Abstract
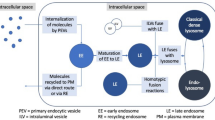
The subcellular distribution of doxorubicin was evaluated in living non-fixed LLC-PK1 cells, which maintain the structural and functional characteristics of the kidney proximal tubule epithelium and also express P-glycoprotein. After 10 min incubation, doxorubicin fluorescence was detectable in the nucleus. The intensity of nuclear fluorescence progressively increased, reaching the maximum at the end of the first hour. Then, the nuclear signal started to decrease and, at 2 h, doxorubicin fluorescence disappeared almost completely from the cell nucleus. Cytoplasmic fluorescent vesicles first appeared in the perinuclear region after 10 min doxorubicin exposure and increased in number and size over a period of 2 h. From 2 to 5 h, fluorescent vesicles moved unidirectionally to the cell periphery. Disappearance of doxorubicin punctate fluorescence in LLC-PK1 cells treated with methylamine or monensin demonstrated that drug accumulation occurred inside acidic compartments. In addition, the cytoplasmic pattern of doxorubicin fluorescence was very similar to that observed upon exposure to the acidotropic tracer LysoSensor Blue. Involvement of P-glycoprotein in doxorubicin handling by LLC-PK1 cells was suggested by modified intracellular doxorubicin distribution after cell incubation with verapamil and vinblastine. Moreover, the fluorescent P-glycoprotein substrate Bodipy FL Verapamil was shown to accumulate in LLC-PK1 cells in a manner that is quite similar to that observed for doxorubicin. P-glycoprotein expression was evaluated by immunoblot using the JSB-1 and C219 monoclonal antibodies. Immunofluorescence analysis was performed using the JSB-1 monoclonal antibody. P-glycoprotein immuno-reactivity was found both on the plasma membrane and intracytoplasmically in a perinuclear position. Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis revealed that MDR1 gene was expressed. This study indicates that a rapid intracellular redistribution accompanies the process of doxorubicin uptake by LLC-PK1 cells. Although these cells are non-tumour cells derived from the normal epithelium of the proximal renal tubule, they display a model of doxorubicin redistribution which is characteristic of doxorubicin-resistant tumour cells.
Similar content being viewed by others
References cited
Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM (1999) Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol 39: 361-398.
Bachur NR, Moore AL, Bernstein JG, Liu A (1970) Tissue distribution and disposition of daunomycin (NSC-82151) in mice: fluorometric and isotopic methods. Cancer Chemother Rep 54: 89-94.
Baldini N, Scotlandi K, Serra M, Shikita T, Zini N, Ognibene A, Santi S, Ferracini R, Maraldi NM (1995) Nuclear immunolocalization of P-glycoprotein in multidrug-resistant cell lines showing similar mechanisms of doxorubicin distribution. Eur J Cell Biol 68: 226-239.
Chomczynski P, Saccho N (1987) Single step method of RNA isolation by acid guanidinium thiocyanate—phenol chloroform extraction. Anal Biochem 162: 156-159.
Coley HM, Amos WB, Twentyman RR, Workman P (1993) Examination by laser scanning confocal fluorescence imaging microscopy of the subcellular localization of anthracyclines in parent and multidrug resistant cell lines. Br J Cancer 67: 1316-1323.
Cordon-Cardo C, O'Brien JP, Boccia J, Casals D, Bertino JR, Melamed MR (1990) Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J Histochem Cytochem 38: 1277-1287.
Decorti G, Peloso I, Favarin D, Bartoli Klugmann F, Candussio L, Crivellato E, Mallardi F, Baldini L (1998) Handling of doxorubicin by the LLC-PK1 kidney epithelial cell line. J Pharmacol Exp Ther 286: 525-530.
De Lange JH, Schipper NW, Schuurhuis GJ, Ten Kate TK, Van Heijningen TH, Pinedo HM, Lnkelma J, Baak JP (1992) Quantification by laser scan microscopy of intracellular doxorubicin distribution. Cytometry 13: 571-576.
Georges E, Bradley G, Gariepy J, Ling V (1990) Detection of P-glycoprotein isoforms by gene-specific monoclonal antibodies. Proc Natl Acad Sci USA 87: 152-156.
Gottesman MM, Pastan I (1993) Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem 62: 385-427.
Handler JS, Perkins FM, Johnson JP (1980) Studies of renal cell function using cell culture techniques. Am J Physiol 238: F1-F9.
Harringan PR, Wong KF, Redelmeier TE, Wheeler JJ, Cullis PR (1993) Accumulation of doxorubicin and other lipophilic amines into large unilamellar vesicles in response to transmembrane pH gradient. Biochim Biophys Acta 1149: 329-338.
Horio M, Pastan I, Gottesman MM, Handler JS (1990) Transepithelial transport of vinblastine by kidney-derived cell lines. Application of a new kinetic model to estimate the in situ Km of the pump. Biochim Biophys Acta 1027: 116-122.
Hull RN, Cherry WR, Weaver GW (1976) The origin and characteristics of a pig kidney cell strain, LLC-PK1. In Vitro 12: 670-677.
Kane SE (1996) Multidrug resistance of cancer cells. Adv Drug Res 28: 181-252.
Kastner S, Wilks MF, Soose M, Bach PH, Stolte H (1991) Metabolic studies on isolated glomeruli: a valuable tool to investigate glomerular damage. In: Bach PH, Gregg NJ, Wilks MW, Delacruz L eds. Nephrotoxicity: Mechanisms, Early Diagnosis and Therapeutic Management. New York: Marcel Dekker, pp. 467-474.
Kastner S, Wilks MF, Gwinner W, Soose M, Bach PH, Stolte H (1990) Metabolic heterogeneity of isolated cortical and juxtamedullary glomeruli in adriamycin nephropathy. Renal Physiol Biochem 14: 48-54.
Krishan A, Sauerteig A, Stein JH (1991) Comparison of the three commercially available antibodies for flow cytometric monitoring of P-glycoprotein expression in tumor cells. Cytometry 12: 731-742.
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685.
Landwehr DM, Carvalho JS, Oken DE (1977) Micropuncture studies of the filtration and absorption of albumin by nephrotic rats. Kidney Int 11: 9-17.
Lelong IH, Guzikowski AP, Haugland RP, Pastan I, Gottesman MM, Willingham MC (1991) Fluorescent verapamil derivative for monitoring activity of the multidrug transporter. Mol Pharmacol 40: 490-494.
Lowry OH, Rosenbrough NJ, Fark AL, Randell LJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275.
Madden TD, Redelmeier TE (1994) Transmembrane distribution of lipophilic cations in response to an electrochemical potential in reconstituted cytochrome c oxidase vesicles and in vesicles exhibiting a potassium ion diffusion potential. J Bioenerg Biomemb 26: 221-230.
Mellman I, Fuchs R, Helenius A (1986) Acidification of the endocytic and exocytic pathways. Annu Rev Biochem 55: 663-700.
Meschini S, Molinari A, Calcabrini A, Citro G, Arancia G (1994) Intra-cellular localization of the antitumor drug adriamycin in living cultured cells: a confocal microscopy study. J Microsc 176: 204-210.
Millot C, Millot JM, Morjani H, Desplaces A, Manfait M (1997) Characterization of acidic vesicles in multidrug-resistant and sensitive cancer cells by acridine orange staining and confocal microspectrofluorimetry. J Histochem Cytochem 45: 1255-1264.
Molinari A, Cianfriglia M, Meschini S, Calcabrini A, Arancia G (1994) P-glycoprotein expression in the Golgi apparatus of multidrugresistant cells. Int J Cancer 59: 789-795.
Molinari A, Calcabrini A, Meschini S, Stringano A, Del Bufalo D, Cianfriglia M, Arancia G (1998) Detection of P-glycoprotein in the Golgi apparatus of drug-untreated human melanoma cells. Int J Cancer 75: 885-893.
Noonan KE, Beck C, Holzmayer TA, Chin JE, Wunder S, Andrulis IL, Gazdar AF, Willman CL, Griffith B, Von Hoff DD, Roninson IB (1990) Quantitative analysis of MDR1 (multidrug resistance) gene expression in human tumors by polymerase chain reaction. Proc Natl Acad Sci USA 87: 7160-7164.
Rutherford AV, Willingham MC (1993) Ultrastructural localization of daunomycin in multidrug-resistant cultured cells with modulation of the multidrug transporter. J Histochem Cytochem 41: 1573-1577.
Seidel A, Hasmann M, L¨oser R, Bunge A, Schefer B, Herzig I, Steidtmann K, Dietel M (1995) Intracellular localization, vesicular accumulation and kinetics of daunorubicin in sensitive and multidrug-resistant gastric carcinoma EPG85-257 cells. Virchows Archiv 426: 249-256.
Scheper RJ, Bulte JW, Brakkee JG, Quak JJ, Van Der Schoot E, Balm AJ, Meijer CJ, Broxterman HJ, Kuiper CM, Lankelma J, Pinedo HM (1988) Monoclonal antibody JSB-1 detects a highly conserved epitope on the P-glycoprotein associated multi-drug resistance. Int J Cancer 42: 389-394.
Simon SM, Schindler M (1994) Cell biological mechanisms of multidrug resistance in tumors. Proc Natl Acad Sci USA 91: 3497-3504.
Sugawara I, Kataoka I, Morishita T, Hamada H, Tsuruo T, Itoyama S, Mori S (1988) Tissue distribution of P-glycoprotein encoded by a multidrug-resistant gene as revealed by a monoclonal antibody, MRK 16. Cancer Res 48: 1926-1929.
Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC(1987) Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA 84: 7735-7738.
Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC (1989) Immunohistochemical localization in normal tissues of different epitopes in the multidrug transport protein P170: evidence for localization in brain capillaries and crossreactivity of one antibody with a muscle protein. J Histochem Cytochem 37: 159-164.
Willingham MC, Richert ND, Corwell MM, Tsuruo T, Hamada H, Gottesman MM, Pastan IH (1987) Immunocytochemical localization of P170 at the plasma membrane of multidrug-resistant human cells. J Histochem Cytochem 35: 1451-1456.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Crivellato, E., Candussio, L., Rosati, A.M. et al. Kinetics of Doxorubicin Handling in the LLC-PK1 Kidney Epithelial Cell Line is Mediated by Both Vesicle Formation and P-glycoprotein Drug Transport. Histochem J 31, 635–643 (1999). https://doi.org/10.1023/A:1003893218761
Issue Date:
DOI: https://doi.org/10.1023/A:1003893218761