Abstract
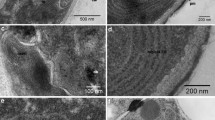
An integrated approach to acid phosphatase (EC 3.1.3.2) histochemistry by the azo-dye and lead-capture (‘Gomori’) methods in phosphate-starved hyphae of the fungus Botrytis cinerea revealed strikingly different patterns of localization of activity staining. Reaction product formed with the azo-dye method was found in numerous small organelles (<;0.5 µm diameter), which also accumulated the lipophilic dye Nile Red and mislocalized the formazan indicating mitochondrial succinate dehydrogenase activity. Such small organelles were stained only weakly and sporadically with the lead-capture method; instead, lead phosphate deposits were produced mainly in large vacuoles (up to 2.5 µm diam.), similar to those accumulating the vital dye Neutral Red. Additionally, acid phosphatase activity was detected in apical secretory vesicles with the lead-capture method but not with the azo-dye method. Ultrastructural studies by transmission electron microscopy confirmed the presence of large vacuoles which showed evidence of autophagic activity, and of small moderately osmiophilic organelles. The latter are considered to be spherosomes rather than lysosomes because of their weak reaction with the lead-capture method and their high lipid content. It is suggested that their apparently strong reaction with the azo-dye method is caused partly by false localization due to the lipophilic nature of the reaction product.
Similar content being viewed by others
References cited
Allison AC, Young MR (1969) Vital staining and fluorescence microscopy of lysosomes. In Lysosomes in Biology and Pathology, Volume 2 (edited by Dingle JT, Fell HB), pp. 600-628. Amsterdam, London: North-Holland.
Armentrout VN, Smith GC, Wilson CL (1968) Spherosomes and mitochondria in the living fungal cell. Am J Bot 55, 1062-1067.
Baba M, Takeshige K, Baba N, Ohsumi Y (1994) Ultrastructural analysis of the autophagic process in yeast: detection of autophagosomes and their characterization. J Cell Biol 124, 903-913.
Beever RE, Burns DJW (1980) Phosphorus uptake, storage and utilization by fungi. Adv Bot Res 8, 127-219.
Boller T, Wiemken A (1986) Dynamics of vacuolar compartmentation. Annu Rev Plant Physiol 37, 137-164.
Bonfante P, Balestrini R, Mendgen K (1994) Storage and secretion processes in the spore of Gigaspora margarita Becker & Hall as revealed by high-pressure freezing and freeze-substitution. New Phytol 128, 93-101.
Buvat R (1977) Origine golgienne et lytique des vacuoles dans les cellulesm éristématique des racines d'Orge (Hordeum sativum). CR Hebd Seances Acad Sci Ser D 284, 167-170.
Chayen J, Bitensky L (1991) Practical Histochemistry. 2nd edition. Chichester: John Wiley & Sons.
Clayton MN, Ashburner CM (1994) Secretion of phenolic bodies following fertilization in Durvillaea potatorum (Durvillaeales, Phaeophyta). Eur J Phycol 29, 1-9.
Cramer CL, Davis RH (1984) Polyphosphate-cation interaction in the amino acid-containing vacuole of Neurospora crassa. J Biol Chem 259, 5152-5157.
Cramer CL, Vaughn LE, Davis LH (1980) Basic amino acids and inorganic polyphosphates in Neurospora crassa: Independent regulation of vacuolar pools. J Bacteriol 142, 945-952.
Frey-Wyssling A, Mühlethaler K (1965) Ultrastructural Plant Cytology Amsterdam: Elsevier Publishing Co.
Gahan PB (1965) Histochemical evidence for the presence of lysosomelike particles in root meristem cells of Vicia faba. J Exp Bot 16, 350-355.
Glauert AM (1974) Fixation, dehydration and embedding of biological specimens. In Practical Methods in Electron Microscopy, Volume 3 (edited by Glauert AM), pp. 1-207. Amsterdam, Oxford: North-Holland.
Gomori G (1950) An improved histochemical technic for acid phosphatase. Stain Technol 25, 81-85.
Greenspan P, Mayer EP, Fowler SD (1985) Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol 100, 965-973.
Grogg E, Pearse AGE (1952) A critical study of the histochemical techniques for acid phosphatase, with a description of an azo dye method. J Pathol Bacteriol 64, 627-636.
Grove SN (1978) The cytology of hyphal tip growth. In The Filamentous Fungi, Volume 3: Developmental Mycology (edited by Smith JE, Berry DR), pp. 28-50. London: E. Arnold.
Hänssler G, Maxwell DP, Maxwell MD (1975) Demonstration of acid phosphatase-containing vacuoles in hyphal tip cells of Sclerotium rolfsii. J Bacteriol 124, 997-1006.
Hänssler G, Maxwell DP, Barczewski H, Bernhardt E (1977) Cytochemische Lokalisation der sauren Phosphatase in Hyphen von Pythium paroecandrum, Botrytis cinerea und Rhizoctonia solani. Phytopathol Z 88, 289-298.
Hislop EC, Barnaby VM, Shellis C, Laborda F (1974) Localization of α-L-arabinofuranosidase and acid phosphatase in mycelium of Sclerotinia fructigena. J Gen Microbiol 81, 79-99.
Holcomb GE, Hildebrandt AC, Evert RF (1967) Staining and acid phosphatase reactions of spherosomes in plant tissue culture cells. Am J Bot 54, 1204-1209.
Holland RD, Pitt D, Moore MN, Brownlee C (1997) Characterization of the egg vesicular components in the seaweed, Fucus serratus L. (Fucales, Phaeophyta), using enzyme histochemistry and vital staining: the search for a lysosome-like body. Histochem J 29, 239-248.
Holt SJ (1954) A new approach to the cytochemical localization of enzymes. Proc Roy Soc Lond B 142, 160-169.
Holtzman E (1989) Lysosomes. New York, London: Plenum Press
López-Franco R, Bartnicki-Garcia S, Bracker CE (1994) Pulsed growth of fungal hyphal tips. Proc Natl Acad Sci USA 91, 12228-12232.
Lüllmann-Rauch R (1979) Drug-induced lysosomal storage disorders. In Lysosomes in Biology and Pathology, Volume 6 (edited by Dingle JT, Jacques PJ, Shaw HI), pp. 49-129. Amsterdam, New York: Elsevier.
Marty F (1978) Cytochemical studies on GERL, provacuoles, and vacuoles in root meristematic cells of Euphorbia. Proc Natl Acad Sci USA 78, 852-856.
Matile P, Moor H (1968) Origin and development of the lysosomal apparatus in root-tip cells. Planta (Berl.) 80, 159-175.
Mims CW, Richardson EA, Clay RP, Nicholson RL (1995) Ultrastructure of conidia and the conidium aging process in the plant-pathogenic fungus Colletotrichum graminicola. Int J Plant Sci 156, 9-18.
Novikoff AB, Holtzman E (1970) Cells and Organelles. London: Holt, Rineheart & Winston.
Parish RW (1975) The lysosome-concept in plants: I. Peroxidases associated with subcellular andwall fractions of maize root tips: Implications for vacuole development. Planta (Berl.) 123, 1-13.
Pitt D (1968) Histochemical demonstration of certain hydrolytic enzymes within cytoplasmic particles of Botrytis cinerea Fr. J Gen Microbiol 52, 67-75.
Pitt D (1975) Lysosomes and Cell Function. London, New York: Longman.
Pitt D, Coombes C (1968) The disruption of lysosome-like particles of Solanum tuberosum cells during infection by Phytophthora erythroseptica Pethybr. J Gen Microbiol 53, 197-204.
Pitt D, Galpin M (1973) Isolation and properties of lysosomes from darkgrown potato shoots. Planta (Berl.) 109, 233-258.
Pitt D, Walker PJ (1967) Particulate localization of acid phosphatase in fungi. Nature (Lond.) 215, 783-784.
Reynolds ES (1963) The use of lead citrate at high pH as an electronopaque stain in electron microscopy. J Cell Biol 17, 208-212.
Sorokin HP (1967) The spherosomes and the reserve fat in plant cells. Am J Bot 54, 1008-1016.
Spurr AR (1969) A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26, 31-43.
Stewart P, Pitt D (1977) Ultrastructural localization of acid phosphatase activity in root tips by the p-(acetoxymercuric) aniline diazotate reagent and a comparison with a Gomori procedure. J Cell Sci 26, 19-29.
Swanson J, Floyd GL (1979) Acid phosphatase in Asteromonas gracilis (Chlorophyceae, Volvocales): a biochemical and cytochemical characterization. Phycologia 18, 362-368.
Takeshige K, Baba M, Tsuboi S, Noda T, Ohsumi Y (1992) Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol 119, 301-311.
Tuttle DL, Dunn WA (1995) Divergent modes of autophagy in the methylotrophic yeast Pichia pastoris. J Cell Sci 108, 25-35.
Wanner G, Formanek H, Theimer RR (1981) The ontogeny of lipid bodies (spherosomes) in plant cells. Planta (Berl.) 151, 109-123.
Weber RWS (1996) Biochemical, physiological and histochemical aspects of acid phosphatase secretion by Botrytis cinerea Pers.:Fr. Ph.D. Thesis, University of Exeter, UK.
Weber RWS, Pitt D (1997a) Acid phosphatase secretion by Botrytis cinerea. Mycol Res 101, 349-356.
Weber RWS, Pitt D (1997b) Purification, characterization and exit routes of two acid phosphatases secreted by Botrytis cinerea. Mycol Res 101, 1431-1439.
Weber RWS, Pitt D (2000) Teaching techniques for mycology: 10. Riddell's slide cultures. Mycologist 14 (in the press).
Weber RWS, Wakley GE, Pitt D (1998) Histochemical and ultrastructural characterization of fungal mitochondria. Mycologist 12, 174-179.
Wilson CL (1973) A lysosomal concept for plant pathology. Annu Rev Phytopathol 11, 247-272.
Wilson CL, Stiers DL, Smith GG (1970) Fungal lysosomes or spherosomes. Phytopathology 60, 216-227.
Wilson CL, Jumper GA, Mason DL (1978) Acridine orange as a lysosome marker in fungal spores. Phytopathology 68, 1564-1567.
Wilson CL, Jumper GA, Mason DL (1980) Vacuole dynamics in fungal plant pathogens. Phytopathology 70, 783-788.
Yatsu LY, Jacks TJ (1972) Spherosome membranes. Half unitmembranes. Plant Physiol 49, 937-943.
Ziegler H (1953) Über die Reduktion des Tetrazoliumchlorids in der Pflanzenzelle und über den Einfluß des Salzes auf Stoffwechsel und Wachstum. Z Naturforsch 8 B, 662-667.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Weber, R.W., Wakley, G.E. & Pitt, D. Histochemical and Ultrastructural Characterization of Vacuoles and Spherosomes as Components of the Lytic System in Hyphae of the Fungus Botrytis cinerea. Histochem J 31, 293–301 (1999). https://doi.org/10.1023/A:1003713901179
Issue Date:
DOI: https://doi.org/10.1023/A:1003713901179