Abstract
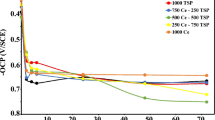
This study investigates corrosion inhibition of steel using thiourea and cations such as aluminium, calcium and magnesium under cathodic control in a 3.5% NaCl solution and in seawater. Steel protection in a 3.5% NaCl solution is normally incomplete under a cathodic potential less electronegative than −1.100 V. However, the protection can be enhanced by nearly 50% by adding either 50 ppm aluminium ion or 75 ppm thiourea in solution, and by almost 90% by the combined use of these additions. This study also analyzes how combining inhibitors and cathodic control may be used to protect steel. Moreover, this investigation monitors pH in the solution, measures zero-resistance current between the graphite-steel couple, as well as analyses cathode reaction products. A mechanism is also proposed to interpret the combined effects of inhibitors and cathodic control on the protection of steel.
Similar content being viewed by others
References
J.C. Lin and H.C. Shih, Corros. Sci. 27 (1987), 839.
J.C. Lin and H.C. Shih, J. Electrochem. Soc. 134 (1987), 817.
C.C. Nathan, Corrosion Inhibitors, 5th Ed., NACE, Houston, Texas, 1981.
I.L. Rozenfeld, Corrosion Inhibitors, McGraw-Hill Inc., New York, 1981.
J.H. Morgan, Cathodic Protection, The Macmillan Company, New York, 1960.
J.T. Reding and J.J. Newport, Materials Protection, December (1966) 15.
R.A. Hine and M.W. Wei, Materials Protection, November (1964) 49.
W.J. Schwerdtfeger, Materials Protection, November (1964) 15.
F.A. Champion, Corrosion Testing Procedures, 2nd Ed., Chapman and Hall, London, 1964 p. 47.
J.C. Lin, H.C. Shih and Y.S. Chang, Proceedings of the 3rd International Electrochemistry Symposium, April, 1986, Taipei, Taiwan, p. 179.
Y.S. Chang and J.C. Lin, Materials Chemistry and Physics, 16 (1986) 31–44
G. Yamaguchi and W.C. Chiu, Bull. Chem. Soc. Jap. 41 (1979) 348.
W.D. Kumler ad G.M. Fohlen, J. Amer. Chem. Soc., 64 (1942) 1944.
R.B. Dean, Modern Colloids, Van Nostrand, New York, 1948 p. 241.
J.A. Dean (Editor), Lange's Handbook of chemistry, 11th Ed., McGraw-Hill, New York, 1974 pp. 3–119.
U.R. Evans, A Introduction to Metallic Corrosion, 3rd Ed. Thomas, East Kilbride, Scotland, 1981 p. 191.
D.A. Jones, Principles and Prevention of Corrosion, Macmillan, New York, 1992 p. 91, p. 360.
R. Remy, in Treatise on Inorganic Chemistry, Ed. by J. Kleinberg, translated by J.S. Anderson, F.R.S., Elsevier, Amsterdam-London, Vol. 1, 1961 p. 351.
J.A. Dean (Editor), Lange's Handbook of chemistry, 11th Ed., McGraw-Hill, New York, 1974 p. 5–7.
R.S. Alwitt, in Oxides and Oxide Films, ed. by J. W. Diggle and A. K. Vijk, Dekker, New York, Vol. 4, 1976 p. 183.
J.J. Fripiat, H. Bosmans and P.G. Rouxhet, J. Phys. Chem. 71 (1967) 1097.
O. Glemser and G. Rieck, Angrew. Chem. 68 (1956) 182.
B. Donnelly, T.C. Downie, R. Grzeskowiak, H.R. Hamburg and D. Short, Corros. Sci. 14 (1974) 597.
G. Oakes and J.M. West, Br. Corros. J. 4 (1969) 66.
T.P. Hoar and R.D. Holliday, J. Appl. Chem. 3 (1953) 502.
H.H. Uhlig, Corrosion and Corrosion Control, 2nd Ed., Wiley, New York, 1971 p. 115.
C. Brosset, Acta Chem. Scand. 6 (1952) 910.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lin, JC., Chang, SL. & Lee, SL. Corrosion inhibition of steel by thiourea and cations under incomplete cathodic protection in a 3.5% NaCl solution and seawater. Journal of Applied Electrochemistry 29, 911–918 (1999). https://doi.org/10.1023/A:1003533800038
Issue Date:
DOI: https://doi.org/10.1023/A:1003533800038