Abstract
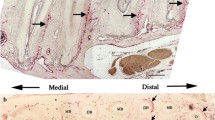
The study assessed immunohistochemically the location and distribution of various non-collagenous matrix proteins (fibronectin, laminin, tenascin-C, osteocalcin, thrombospondin-1, vitronectin and undulin) in musculoskeletal tissues of rat. Fibronectin and thrombospondin-1 were found to be ubiquitous in the studied tissues. High immunoreactivity of these proteins was found in the extracellular matrix of the anatomical sites where firm bindings are needed, i.e. between muscle fibres and fibre bundles, between the collagen fibres of a tendon and at myotendinous junctions, osteotendinous junctions and articular cartilage. Tenascin-C was found in the extracellular matrix of regions where especially high forces are transmitted from one tissue component to the other, such as myotendinous junctions and osteotendinous junctions. Laminin was demonstrated in the basement membranes of the muscle cells and capillaries of the muscle–tendon units. Osteocalc in immunoreactivity concentrated in the extracellular matrix of areas of newly formed bone tissue, i.e. in the subperiosteal and subchondral regions, osteoid tissue and mineralized fibrocartilage zone of the osteotendinous junction. Mild vitronectin activity could be seen in the extracellular matrix of the osteotendinous and myotendinous junctions, and high activity around the bone marrow cells. Undulin could be demonstrated in the extracellular matrix (i.e. on the collagen fibres) of the tendon and epimysium only. However, it was co-distributed with fibronectin and tenascin-C. Together, these findings on the normal location and distribution of these non-collagenous proteins in the musculoskeletal tissues help to form the basis of knowledge against which the location and distribution of the these proteins in various pathological processes could be compared.
Similar content being viewed by others
References
Bauer, M., Dieterich, W., Ehnis, T. & Schuppan, D. (1997) Complete primary structure of human collagen type XIV. Biochim. Biophys. Acta 1354, 183-8.
Bornstein, P. (1992) Thrombospondins: structure and regulation of expression. FASEB J. 6, 3290-9.
Bornstein, P. (1995) Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J. Cell Biol. 130, 503-6.
Bornstein, P. & Sage, E.H. (1994) Thrombospondins. Methods Enzymol. 245, 62-84.
Chevalier, X., Groult, N., Larget-piet, B., Zardi, L. & Hornebeck, W. (1994) Tenascin distribution in articular cartilage from normal subjects and from patients with osteoarthritis and rheumatoid arthritis. Arthr. Rheum. 37, 1013-22.
Chiquet, M. & Fambrough, D.M. (1984) Chick myotendinous antigen. I. Monoclonal antibody as a marker for tendon and muscle morphogenesis. J. Cell Biol. 98, 1926-36.
Chiquet-ehrismann, R. (1990) What distinguishes tenascin from Fibronectin? FASEB J. 4, 2598-604.
Chiquet-ehrismann, R. (1995) Tenascins, a growing family of extracellular matrix proteins. Experientia 51, 853-62.
Chiquet-ehrismann, R., Kalla, P., Pearson, C.A., Beck, K. & Chiquet, M. (1988) Tenascin interferes with Fibronectin action. Cell 53, 383-90.
Chiquet-ehrismann, R., Tannerheim, M., Koch, M., Brunner, A., Spring, J., Martin, D., Baumgartner, S. & Chiquet, M. (1994) Tenascin-C expression by Fibroblasts is elevated in stressed collagen gels. J. Cell Biol. 127, 2093-101.
Chuong, C.M., Widelitz, R.B., Jiang, T.X., Abbott, L.P.K., Lee, Y.S. & Chen, H.M. (1993) Roles of adhesion molecules NCAM and tenascin in limb skeletogenesis: analysis with antibody pertubation, exogenous gene expression, talpid mutants and active stimulation. Prog. Clin. Biol. Res. 383B, 465-74.
Cockburn, C.G. & Barnes, M. J. (1991) Characterization of thrombospondin binding to collagen (type I) Fibres: role of collagen telopeptides. Matrix 11, 168-76.
Ducy, P., Desbois, C., Boyce, B., Pinero, G., Story, B., Dunstan, C., Smith, E., Bonadio, J., Goldstein, S., Gundberg, C., Bradley, A. & Karsenty, G. (1996) Increased bone formation in osteocalcin-deFIcient mice. Nature 382, 448-52.
Ehnis, T., Dieterich, W., Bauer, M. Kresse, H. & Schuppan, D. (1997).Localization of a binding site for the proteoglycan decorin on collagen XIV (undulin). J. Biol. Chem. 272, 20414-19.
Erickson, H.P. (1993) Tenascin-C, tenascin-R and tenascinX: a family of talented proteins in search of functions. Curr. Opin. Cell Biol. 5, 869-76.
Frazier, W.A. (1987) Thrombospondin: a modular adhesive glycoprotein of platelets and nucleated cells. J. Cell Biol. 105, 625-32.
Garnero, P., Grimaux, M., Seguin, P. & Delmas, P.D. (1994) Characterization of immunoreactive forms of human osteocalcin generated in vivo and in vitro. J. Bone Miner. Res. 9, 255-64.
Gebb, C., Hayman, E.G., Engvall, E. & Ruoslahti, E. (1986) Interactions of vitronectin with collagen. J. Biol. Chem. 261, 16698-703.
Glumoff, V., Savontaus, M., Vesanen, J. & Vuorio, E. (1994) Analysis of aggrecan and tenascin gene expression in mouse skeletal tissues by Northern and in situ hybridization using speciFIc cDNA probes. Biochim. Biophys. Acta 1219, 613-22.
Grzesik, W. & Gehron-robey, P. (1994) Bone matrix RGD glycoproteins: immunolocalization and interaction with human primary osteoblastic bone cells in vitro. J. Bone Miner. Res. 9, 487-96.
Horton, M.A., Dorey, E.L., Nesbitt, S.A., Samanen, J., Ali, F.E., Stadel, J.M., Nichols, A., Greig, R. & Helfrich, M.H. (1993) Modulation of vitronectin receptor-mediated osteoclast adhesion by Arg-glyasp peptide analogs: a structure-function analysis. J. Bone Miner. Res. 8, 239-47.
Hurme, T. (1991) Regeneration of injured skeletal muscle: an experimental study with rats. Academic dissertation, Medica-Odontologica, series D, vol. 82. University of Turku, Turku, Finland.
Hurme, T. & Kalimo, H. (1992) Adhesion in skeletal muscle during regeneration. Muscle Nerve 15, 482-9.
Hynes, R.O. (1986) Fibronectins. Sci. Am. 254, 42-51.
Ingram, R.T., Park, Y-K., Clarke, B.L. & Fitzpatrick, L.A. (1994) Age-and gender-related changes in the distribution of osteocalcin in the extracellular matrix of normal male and female bone. J. Clin. Invest. 93, 989-97.
Irintchev, A., Salvini, T.F., Faissner, A. & Wernig, A. (1993) Differential expression of tenascin after denervation, damage or paralysis of mouse soleus muscle. J. Neurocytol. 22, 955-65.
JÄrvinen, M., Kannus, P., Kvist, M., Isola, M J., Lehto, M. & Jozsa, L. (1991) Macromolecular composition of the myotendinous junction. Exp. Mol. Pathol. 55, 230- 7.
Jozsa, L. & Kannus, P. (1997) Human Tendons. Anatomy, Physiology, and Pathology. Champaign, IL, USA: Human Kinetics.
Jozsa, L., Lehto, M., Kannus, P., Kvist, M., Reffy, A., Vieno, T., JÄrvinen, M., Demel, S. & Elek, E. (1989) Fibronectin and laminin in Achilles tendon. Acta Orthop. Scand. 60, 469-71.
Jozsa, L., Kvist, M., Kannus, P., Vieno T., JÄrvinen, M. & Lehto, M. (1991) Structure and macromolecular composition of the myotendinous junction. Acta Morphol. Hung. 39, 287-97.
Just, M., Herbst, H., Hummel, M., DÜrkop, H.T., Tri-pier, D., Stein, H. & Schuppan, D. (1991) Undulin is a novel member of the Fibronectin-tenascin family of extracellular matrix glycoproteins. J. Biol. Chem. 266, 17326-32.
Kannus, P., Jozsa, L., Kvist, M., JÄrvinen, T.L.N., Maunu, V-M., Hurme, T. & JÄrvinen, M. (1996) Expression of osteocalcin in the patella of experimentally immobilized and remobilized rats. J. Bone Miner. Res. 11, 79-87.
Kvist, M., Jozsa, L., Kannus, P., Isola, J., Vieno, T., JÄrvinen, M. & Lehto, M. (1991) Morphology and histochemistry of the myotendineal junction of the rat calf muscles. Acta Anat. 141, 199-205.
Lawler, J.W. (1986) Review: the structural and functional properties of thrombospondin. Blood 67, 1192-204.
Lehto, M., Duance, V.C. & Restall, D. (1985) Collagen and Fibronectin in the healing skeletal muscle injury. J. Bone Joint Surg. 66B, 820-8.
Lehto, M., Kvist, M., Vieno, T. & Jozsa, L. (1988) Macromolecular composition of the sarcolemma and endomysium in the rat. Acta Anat. 133, 297-302.
Mackie, E. J. & Ramsey, S. (1996) Expression of tenascin in joint-associated tissues during development and postnatal growth. J. Anat. 188, 157-65.
Miller, R.R. & Mcdevitt, C.A. (1988) Thrombospondin is present in articular cartilage and is synthesized by articular chondrocytes. Biochem. Biophys. Res. Commun. 153, 708-14.
Neurath, M. (1993) Expression of tenascin, laminin and Fibronectin following traumatic rupture of the anterior cruciate ligament. Z. Orthop. 131, 168-72.
Neurath, M.F. & Stofft, E. (1992) Structure and function of matrix components in the cruciate ligaments. Acta Anat. 145, 387-94.
Reilly, J.T. & Nash, J.R.G. (1988) Vitronectin (serum spreading factor): its localisation in normal and FIbrotic tissue. J. Clin. Pathol. 41, 1269-72.
Risteli, L. & Risteli, J. (1993) Biochemical markers of bone metabolism. Ann. Med. 25, 385-93.
Roberts, D.D. (1996) Regulation of tumor growth and metastasis by thrombospondin-1. FASEB J. 10, 1183- 91.
Robey, P.G., Young, M.F., Fischer, L.W. & Mcclain, T.D. (1989) Thrombospondin is an osteoblast-derived component of mineralized extracellular matrix. J. Cell Biol. 108, 719-27.
Ruoslahti, E. (1988) Fibronectin and its receptors. Annu. Rev. Biochem. 57, 375-413.
Salter, D.M. (1993) Tenascin is increased in cartilage and synovium from arthritic knees. Br. J. Rheumatol. 32, 780-6.
Schuppan, D., Cantaluppi, M.C., Becker, J., Veit, A., Bunte, T., Troyer, D., Schuppan, F., Schmid, A., Ackermann A. & Hahn, E.G. (1990) Undulin, an extracellular matrix glycoprotein associated with collagen FIbrils. J. Biol. Chem. 265, 8823.
Timpl, R. & Brown, J.C. (1994) The laminins. Minireview. Matrix Biol. 14, 275-81.
WÄlchli, C., Trueb, J., Kessler, B., Winterhalter, K.H. & Trueb, B. (1993).Complete primary structure of chicken collagen XIV. Eur. J. Biochem. 212, 483-90.
Webb, C.M.B., Zaman, G., Mosley, J.R., Tucker, R.P., Lanyon, L.E. & Mackie, E. J. (1997) Expression of tenascin-C in bones responding to mechanical load. J. Bone Miner. Res. 12, 52-8.
Wewer, U. & Engvall, E. (1994) Laminins. Methods Enzymol. 245, 85-104.
Wight, T.N., Raugi, G. J., Mumby, S.M. & Bornstein, P. (1985) Light microscopic immunolocation of thrombospondin in human tissues. J. Histochem. Cytochem. 33, 295-302.
Young, M.F., Kerr, J.M., Ibaraki, K., Heegaard, A.M. & Robey, P.G. (1992) Structure, expression, and regulation of the major noncollagenous matrix proteins of bone. Clin. Orthop. 281, 275- 94.
Zhang, X., Schuppan, D., Becker, J., Reichart, P. & Gelderblom, H.R. (1993) Distribution of undulin, tenascin, and Fibronectin in the human periodontal ligament and cementum: comparative immunoelectron microscopy with ultra-thin cryosections. J. Histochem. Cytochem. 41, 245-51.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kannus, P., Jozsa, L., Järvinen, T.A.H. et al. Location and Distribution of Non-collagenous Matrix Proteins in Musculoskeletal Tissues of Rat. Histochem J 30, 799–810 (1998). https://doi.org/10.1023/A:1003448106673
Issue Date:
DOI: https://doi.org/10.1023/A:1003448106673