Abstract
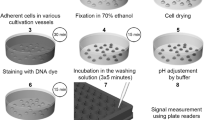
The majority of studies dealing with DNA analyses are made on fixed cells. In this context, the efficiency as fixatives of ethanol, methanol, acetone, Carnoy, Boehm-Sprenger and aldehydes was determined using two different DNA fluorescent probes, Hoechst 33342 and propIDium iodIDe. The purpose of our study was to find the fixative that would provIDe the best results with respect to the following parameters: aggregates, cell size and granularity, and DNA staining analysis. Using murine fibroblasts, we found that 68% ethanol, 85% methanol and aldehydes dID not increase aggregate formation, whereas Carnoy, acetone or Boehm-Sprenger fixatives dID. The results show that aldehydes seem to alter cell size least. All fixatives induce an increase in cell granularity, which is very pronounced with alcohols, but aldehydes alter morphology less than alcohols. We observed that the fixatives giving the best resolution with Hoechst 33342 staining lead to a lower measurement variabili ty than with propIDium iodIDe staining. This study leads us to conclude that 68% ethanol and 85% methanol can be consIDered as appropriate fixatives for flow cytometry studies of DNA content.
Similar content being viewed by others
References
Baisch, H., Gohde, W. & Linden, W.A. (1975) Mathematical analysis of pulse-cytophotometric data to determine the fraction of cells in the various phases of cell cycle. Radiat. Environ. Biophys. 12, 31–9.
Braylan, R.C., Benson, N.A., Nourse, V. & Kruth, H.S. (1982) Correlated analysis of cellular DNA, membrane antigens and light scatter of human lymphoid cells. Cytometry 2, 337–43.
Crissman, H.A. & Steinkamp, J.A. (1973) Rapid simultaneous measurement of DNA, protein, and cell volume in single cells from large mammalian cell populations. J. Cell. Biol. 59, 766–71.
Crissman, H.A. & Steinkamp, J.A. (1990) Cytochemical techniques for multivariate analysis of DNA and other cellular constituents. In: Flow Cytometry and Sorting (edited by M.-R. Melamed, T. Lindmo and M.-L. Mendelsohn), 2nd edn, pp. 227–47. New York: Wiley-Liss.
Crissman, H.A. & Tobey, R.A. (1974) Cell cycle analysis in 20 minutes. Science 184, 1297–8.
Esteban, J.M., Sheibani, K., Owens, M., Joyce, J., Bailey, A. & Battifora, H. (1991) Effects of various fixatives and fixation conditions on DNA ploidy analysis. A need for strict internal DNA standards. Am. J. Clin. Pathol. 95: 460–6.
Gabe, M. (1968). Techniques Histologiques. Paris: Masson.
Herbert, D.J., Nishiyama, R.H., Bagwell, C.B., Munson, M.E., Hitchcox, S.A. & Lovett, E. J. (1989) Effects of commonly used fixatives on DNA and total nuclear protein analysis by flow cytometry. Am. J. Clin. Pathol. 91, 535–41.
Holtfreter, H.B., & Cohen, N. (1990) Fixation-associated quantitative variations of DNA fluorescence observed in flow cytometric analysis of hemopoietic cells from adult diploid frogs. Cytometry 11, 676–85.
Jaccoberger, J.W., Fogleman, D. & Lehman, J.M. (1986) Analysis of intracellular antigens by flow cytometry. Cytometry 7, 356–64.
Krishan, A. (1975) Rapid flow cytophotometric analysis of mammalian cell cycle by propidium iodide staining. J. Cell. Biol. 66, 188–93.
Krishan, A. (1987) Affect of drug efflux blockers on vital staining of cellular DNA with Hoechst 33342. Cytometry 8, 642–5.
Larsen, J.K., Munch-peterson, B., Christiansen, J. & Jorgensen, K. (1986) Flow cytometric discrimination of mitotic cells: resolution of M, as well as G1, S and G2 phase nuclei with mithramycin, propidium iodide and ethidium bromide after fixation with formaldehyde. Cytometry 7, 54–63.
Leitner, F., Paillasson, S., Ronot, X. & Demongeot, J. (1995). Dynamic functional and structural analysis of living cells: vital staining of nuclear DNA for chromatin analysis and characterization of cell migration. Acta Biotheoretica 43, 299–317.
Lepecq, J. B. & Paoletti, C. (1967) A fluorescent complex between ethidium bromide and nucleic acids. Physicalchemical characterization. J. Mol. Biol. 27, 87–106.
Maciorowski Z., Veilleux C., Gibaud A., Bourgeois C.A., Klijanienko J., Boenders J. & Vielh P. (1997) Comparison of fixation procedures for fluorescent quantitation of DNA content using image cytometry. Cytometry 28, 123–9.
Paillasson, S., Millot J.M., Manfait M. & Ronot, X. (1994) DNA analysis in living cells: cytometric approaches. In: Visualization of Nucleic Acids (edited by G. Morel), pp. 137–54. New York: CRC Press.
Petersen, S.E. (1986) Accuracy and reliability of flow cytometric DNA analysis using a simple, one-step ethidium bromide staining protocol. Cytometry 7, 301–6.
Rabinovitch, P.S. (1993) Practical considerations for DNA content and cell cycle analysis. In: Clinical Flow Cytometry, Principles and Applications (edited by K.D. Bauer, E.D. Duque and T.V. Shankey), pp. 117–42. Baltimore: Williams & Wilkins.
Santisteban, M.S., Montmasson, M.P., Giroud, F., Ronot, X. & Brugal, G. (1992) Fluorescence image cytometry of nuclear DNA content versus chromatin pattern: a comparative study of ten fluorochromes. J. Histochem. Cytochem. 40, 1789–97.
Schimenti, K.J. & Jaccoberger, J. (1992) Fixation of mammalian cells for flow cytometric evaluation of DNA content and nuclear immunofluorescence. Cytometry 13, 48–59.
Shapiro, H.M. (1981) Flow cytometric estimation of DNA and RNA content in intact cells stained with Hoechst 33342 and pyronin Y. Cytometry 2, 143–50.
Taylor, I.W. (1980) A rapid single step staining technique for DNA analysis by flow microfluorometry. J. Histochem. Cytochem. 28, 1021–4.
Thornthwhaite, J.T., Sugarbaker, E.V. & Temple, W.J. (1980) Preparation of tissues for DNA flow cytometric analysis. Cytometry 1, 229–37.
Urielli-Shoval, S., Meek, R.L., Hanson, R.H., Ferguson, M., Gordon, D. & Benditt, E. (1992) Preservation of RNA for in situ hybridization: Carnoy's versus formaldehyde fixation. J. Histochem. Cytochem. 40, 1879–85.
Vindelov, L.L., Christensen, I. J. & Nissen, N.I. (1983a) A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry 3, 323–7.
Vindelov, L.L., Christensen, I. J. & Nissen, N.I. (1983b) Standardization of high resolution flow cytometric DNA analysis by the simultaneous use of chicken and trout red blood cells as internal reference standards. Cytometry 3, 328–31.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rousselle, C., Robert-Nicoud, M. & Ronot, X. Flow Cytometric Analysis of DNA Content of Living and Fixed Cells: a Comparative Study Using Various Fixatives. Histochem J 30, 773–781 (1998). https://doi.org/10.1023/A:1003440423316
Issue Date:
DOI: https://doi.org/10.1023/A:1003440423316