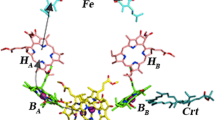
Abstract
Although the two electron-transfer branches in the reaction centers (RC) of purple bacteria are virtually symmetric, it is well known that only one of them is functionally active (the A-branch). The mechanisms of functional asymmetry of structurally symmetric branches of the electron transport system are analyzed in this work within the framework of the theory of bimolecular charge-transfer complexes (CTC). CTC theory is shown to provide an explanation of this phenomenon. According to the CTC theory, the dominance of one branch is required to implement the CTC state in special bacteriochlorophyll pairs of RC, in which more than 30% of the excited electron density in the CTC is shifted toward one of the bacteriochlorophyll molecules. This causes a significant increase in the efficiency of further electron transfer to the primary quinone acceptor as compared to a system with two absolutely symmetric electron transfer branches. Specific features of dielectric asymmetry near the RC special pair are discussed. It is emphasized that a strong CTC is able to provide effective trapping of electronic excitation energy from antenna chlorophyll, which is a main function of the RC. Hypothetical stages of CTC formation in other classes of photosynthesizing bacteria during evolution are discussed.
Similar content being viewed by others
REFERENCES
Williams, J. C., Alden, R. G., Murchison, H. A., Peloquin, J. M., Woodbury, N. W., and Allen, J. P. (1992) Biochemistry, 31, 11029-11037.
Heller, B. A., Holten, D., and Kirmaier, C. (1995) Science, 269, 940-945.
Rautter, J., Lendzian, F., Schultz, C., Fetsch, A., Kuhn, M., Lin, X., Williams, J. C., Allen, J. P., and Lubitz, W. (1995) Biochemistry, 34, 8130-8143.
Taguchi, A. K. W., Eastman, J. E., Gallo, D. M., Jr., Xiao, W., and Woodbury, N. W. (1996) Biochemistry, 35, 3175-3186.
Artz, K., Williams, J. C., Lendzian, J. P., Rautter, J., and Lubitz, W. (1997) Proc.Natl.Acad.Sci.USA, 94, 13582-13587.
Kirmaier, C., and Holten, D. (1993) in The Photosynthetic Reaction Center (Deisenhofer, J., and Norris, J., eds.) Vol. 2, Academic Press, San Diego, pp. 4970.
Woodbury, N. W., and Allen, J. P. (1995) in Anoxygenic Photosynthetic Bacteria (Blankenship, R. E., Madigan, M. T., and Bauer, C. E., eds.) Kluwer Academic Publishers, The Netherlands, pp. 527-557.
Terenin, A. N. (1967) Photonics of Dye Molecules [in Russian], Nauka, Leningrad.
Grant, E. H., Sheppard, R. J., and South, G. H. (1968) Dielectric Behavior of Biological Molecules in Solution, Clarendon Press, Oxford, p. 137.
Davydov, A. N. (1976) The Solid State Theory [in Russian], Naukova Dumka, Kiev.
Van Grondelle, R., Dekker, J. P., Gilbro, T., and Sundstrem, V. (1994) Biochim.Biophys.Acta, 1187, 1-65.
Hoff, A. J. (1993) Molecular Physics, 78, 799-819.
Michel-Beyerle, M. E., Plato, M., Deisenhofer, J., Michel, H., Bixon, M., and Jortner, J. (1988) Biochim.Biophys.Acta, 932, 52-70.
Steffen, M. A., Lao, K., and Boxer, S. G. (1994) Science, 264, 810-816.
Moore, L. J., Zhou, H., and Boxer, S. G. (1999) Biochemistry, 38, 11949-11960.
Parson, W. W., Chu, Z. T., and Warshel, A. (1990) Biochim.Biophys.Acta, 1017, 251-272.
Kirmaier, C., Weems, D., and Holten, D. (1999) Biochemistry, 38, 11516-11530.
Bylina, E. J., and Youvan, D. C. (1988) Proc.Natl.Acad.Sci.USA, 85, 7226-7230.
Shkuropatov, A. Ya., and Shuvalov, V. A. (1993) FEBS Lett., 322, 168-172.
Fok, M. V., and Borisov, A. Y. (1981) Stud.Biophys., 32, 115-124.
Borisov, A. Y., and Fok, M. V. (1999) Biochem.Molec.Biol.Int., 47, 117-125.
Kirmaier, C., Holten, D., Bylina, E. J., and Youvan, D. C. (1988) Proc.Natl.Acad.Sci.USA, 85, 7562-7566.
McDowell, L. M., Gaul, D., Kirmaier, C., Holten, D., and Schenck, C. C. (1991) Biochemistry, 30, 8115-8122.
Ivancich, A., Artz, K., Williams, J. C., Allen, J. P., and Mattioli, T. (1998) Biochemistry, 37, 11812-11820.
Ivancich, A., Mattioli, T., Artz, K., Wang, S., Allen, J. P., and Williams, J. C. (1997) Biochemisrty, 36, 3027-3036.
Sakurai, H., Kusumoto, N., and Injue, K. (1996) Photochem.Photobiol., 64, 5-13.
Tserniker, Y. L., and Chetverikov, A. G. (1988) Photosynthetica, 22, 483-490.
Bolton, J. R. (ed.) (1977) Solar Power and Fuels, Academic Press, N. Y.
Skulachev, V. P. (1989) Energetics of Biological Membranes [in Russian], Nauka, Moscow.
Schubert, W. D., Klukas, O., Kraus, N., Saenger, W., Fromme, P., and Witt, H. T. (1997) J.Mol.Biol., 272, 741-769.
Foerster, T. (1948) Ann.Physik., 2, 55-75.
Ermler, U., Fritzsch, G., Buchanan, S. K., and Michel, H. (1994) Structure, 2, 925-936.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Borisov, A.Y. Why Is Electron Transport in the Reaction Centers of Purple Bacteria Unidirectional?. Biochemistry (Moscow) 65, 1429–1434 (2000). https://doi.org/10.1023/A:1002865109271
Issue Date:
DOI: https://doi.org/10.1023/A:1002865109271