Abstract
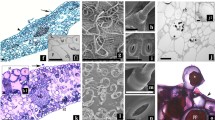
Whole sugar beet (Beta vulgaris L. cv. Ras poly) plants were grown in the greenhouse from the same seed stock used for an in vitro shoot tip culture. In vitro produced sugar beet plants exhibited a high content of chlorophylls a and b, carotene, and total and soluble sugars. On the other hand, total protein content of in vivo plants was higher than that of in vitro plants. No differences were found by SDS-PAGE analysis in the nature and contents of soluble proteins of in vitro propagated plants and greenhouse-grown plants. Surfaces of epidermal cells were larger and palisade and spongy paranchyma tissues were thicker in leaves of regenerants than in leaves of seedlings. Vascular tissues in leaf petioles in regenerants were flat and more differentiated than in seedlings. Closed and undeveloped stomata were found on the abaxial leaf surface of regenerants, whereas in seedlings the stomata were open.
Similar content being viewed by others
References
Bradford, M.M.: A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding.-Ann. Biochem. 72: 248–245, 1976.
Brainerd, K.E., Fuchigami, L.H., Kwiatkowski, S., Clark, C.S.: Leaf anatomy and water stress of aseptically cultured ‘Pixy’ plum grown under different environments.-HortScience 16: 173–175, 1981.
Cocking, E.C., Riley, R.: Application of tissue culture and somatic hybridization of plant improvement.-In: Frey, K.J. (ed.): Plant Breeding II. Pp. 85–116. Iowa State Univ. Press, Ames 1981.
Crèvecoeur, M., Hagège, D., Catesson, A. M., Greppin, H., Gaspar, T.: Ultrastructural characteristics of cells from normal and habituated sugar beet calli.-Plant Physiol. Biochem. 30: 87–95, 1992.
D'Amato, F.: Cytogenetics of plant cell and tissue cultures and their regenerates.-Crit. Rev. Plant Sci. 3: 73–112, 1985.
Donnelly, D.J., Vidaver, W.E.: Pigment content and gas exchange of red raspberry in vitro and ex vitro.-J. amer. Soc. hort. Sci. 109: 177–181, 1984.
Donnelly, D.J., Vidaver, W.E., Lee, K.Y.: The anatomy of tissue cultured red raspberry prior to and after transfer to soil.-Plant Cell Tissue Organ Cult. 4: 43–50, 1985.
Grout, B.W.W.: Wax development on leaf surfaces of Brassica oleracea var. Currawong regenerated from meristem culture.-Plant Sci. Lett. 5: 401–405, 1975.
Grout, B.W.W., Aston, M.J.: Modified leaf anatomy of cauliflower plantlets regenerated from meristem culture.-Ann. Bot. 42: 993–995, 1978.
Herbert, D., Phipps, P.J., Strange, R.E.: Determination of total carbohydrate.-Methods Microbiol. 5B: 209–344, 1971.
Jackson, G.: Crystal violet and erythrosin in plant anatomy.-Stain Technol. 1: 33–34, 1926.
Johanson, D.A.: Plant Microtechnique.-McGraw Hill Brook Co., New York-London 1940.
Krstić, B., Mezei, S., Kovačev, L., Pajević, S.: Chlorophylls and carotenoids in calli of sugar beet genotypes.-Biol. Plant. 38: 621–624, 1996.
Laemmli, U.K.: Cleavage of structural proteins during the assembly of the head bacteriophage T4.-Nature 224: 680–685, 1970.
Lee, N., Wetzstein, H.Y., Sommer, H.E.: Quantum flux density effects on the anatomy and surface morphology of in vitro and in vivo developed sweetgum leaves.-J. amer. Soc. hort. Sci. 113: 167–171, 1988.
Maene, I.J., Debergh, P.C.: Optimization of the transfer of tissue cultured shoots to in vivo conditions.-Acta Hort. 212: 335–348, 1987.
Mezei, S., Jelaska, S., Kovacev, L.: Vegetative propagation of sugar beet from floral ramets.-J. Sugar Beet Res. 27(3–4): 90–96, 1990.
Murashige, T.: Plant propagation through tissue culture.-Annu. Rev. Plant Physiol. 25: 135–166, 1974.
Murashige, T., Skoog, F.: A revised medium for rapid growth and bioassays with tobacco tissue cultures.-Physiol. Plant. 15: 473–497, 1962.
O'Leary, J.W., Knecht, G.N.: Elevated CO2 concentration increases stomata numbers in Phaseolus vulgaris leaves.-Bot. Gaz. 142: 438–441, 1981.
Pospíšilová, J., Čatský, J., Šesták, Z.: Photosynthesis in Plants Cultivated In Vitro.-In: Pessarakli, M. (ed.): Handbook of Photosynthesis. Pp. 525–540. Marcel Dekker, New York-Basel-Hong Kong 1997.
Rady, M.R.: In vitro multiplication of Beta vulgaris L. throughout excised shoot tips.-Biol. Plant. 40: 515–522, 1997/98.
Reuther, G.: Comparative anatomical and physiological studies with ornamental plants under in vitro and greenhouse conditions. Propagation of ornamentals.-Acta Hort. 226: 91–97, 1988.
Saebo, A., Krekling, T., Appelgren, M.: Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro.-Plant Cell Tissue Organ Cult. 41: 177–185, 1995.
Sarić, M., Kasztori, R., Čurić, R., Čupina, T., Gerić, I.: Chlorophyll determination.-In: Univerzitet u Novom Sadu. Praktikum 12: Fiziologija Biljaka. P. 215. Naučna Knjiga, Beograd 1967.
Smith, M.A.L., Palta, J.P., McCown, B.H.: Comparative anatomy and physiology of microcultured, seedling, and greenhouse-grown Asian white birch.-J. amer. Soc. hort. Sci. 111: 437–442, 1986.
Svirshchevskaya, A.M.: [Characteristics of sugar beet plants produced in in vitro culture.]-In: Sovremennye Metody i Podkhody v Selektsii Rasteniï. Pp. 96–101. Kishinev 1991. [In Russ.]
Toldi, O., Gyulai, G., Preininger, E., Várallyay, E., Fári, M., Balázs, E.: Minibeet initiation from derooted sugar beet (Beta vulgaris L.) seedling in vitro.-Plant Sci. 97: 217–224, 1994.
Ultrika, E., Lars, H.M., Sara, V.A.: Extracellular proteins in embryogenic suspension cultures of Norway spruce Picea abies L.-Physiol. Plant. 88: 315–321, 1993.
Wardle, K., Short, K.C.: Stomatal response of in vitro cultured plantlets. Responses in epidermal strips of chrysanthemum to environmental factors and growth regulators.-Biochem. Physiol. Pflanz. 178: 619–624, 1983.
Wetzstein, H.Y., Sommer, H.E.: Leaf anatomy of tissue-cultured Liquidambar styraciflua (Hamamelidaceae) during acclimatization.-Amer. J. Bot. 69: 1579–1586, 1982.
Zhong, Z.X., Smith, H.G., Thomas, T.H.: Micropropagation of wild beet Beta maritima from inflorescence pieces.-Plant Growth Regul. 12: 53–58, 1993.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rady, M., Ali, Z. Comparison of Physiology and Anatomy of Seedlings and Regenerants of Sugar Beet. Biologia Plantarum 42, 39–48 (1999). https://doi.org/10.1023/A:1002107106888
Issue Date:
DOI: https://doi.org/10.1023/A:1002107106888