Abstract
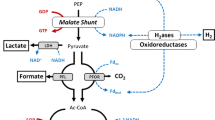
A pathway for conversion of the metabolic intermediate phosphoenolpyruvate (PEP) and the formation of acetate, succinate, formate, and H2 in the anaerobic cellulolytic bacterium Ruminococcus flavefaciens FD-1 was constructed on the basis of enzyme activities detected in extracts of cells grown in cellulose- or cellobiose-limited continuous culture. PEP was converted to acetate and CO2 (via pyruvate kinase, pyruvate dehydrogenase, and acetate kinase) or carboxylated to form succinate (via PEP carboxykinase, malate dehydrogenase, fumarase, and fumarate reductase). Lactate was not formed even during rapid growth (batch culture, µ = 0.35/h). H2 was formed by a hydrogenase rather than by cleavage of formate, and 13C-NMR and14 C-exchange reaction data indicated that formate was produced by CO2 reduction, not by a cleavage of pyruvate. The distribution of PEP into the acetate and succinate pathways was not affected by changing extracellular pH and growth rates within the normal growth range. However, increasing growth rate from 0.017/h to 0.244/h resulted in a shift toward formate production, presumably at the presence of H2. This shift suggested that reducing equivalents could be balanced through formate or H2 production without affecting the yields of the major carbon-containing fermentation endproducts.
Similar content being viewed by others
References
Anonymous (1973) Lactate dehydrogenase. In: Biochemica Information Vol I (pp. 120–121). Boehringer-Mannheim, Mannheim, Germany
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254
Canovas JL & Kornberg HL (1969) Phosphoenolpyruvate carboxylase from Escherichia coli. Methods Enzymol. 13: 288–292
Collins LB & Thomas TD (1974) Pyruvate kinase of Streptococcus lactis. J. Bacteriol. 120: 52–58
Hopgood MF & Walker DJ (1969) Succinic acid production by rumen bacteria. III. Enzymic studies on the formation of succinate by Ruminococcus flavefaciens. Austral. J. Biol. Sci. 22: 1413–1424
Hsu RY & Lardy HA (1969) Malic enzyme. Methods Enzymol 13: 231–235
Hungate RE, Smith W, Bauchop T, Yu I & Rabinowitz JC (1970) Formate as an intermediate in the rumen fermentation. J. Bacteriol. 102: 389–397
Joyner AE Jr & Baldwin RL (1966) Enzymatic studies of pure cultures of rumen microorganisms. J. Bacteriol. 92: 1321–1330
Joyner AE Jr, Winter WE & Godbout DM (1977) Studies on some characteristics of hydrogen production by cell-free extracts of rumen anaerobic bacteria. Can. J. Microbiol. 23: 346–353
Latham MJ & Wolin MJ (1977) Fermentation of cellulose by Ruminococcus flavefaciens in the presence and absence of Methanobacterium ruminantium. Appl. Environ. Microbiol. 34: 297–301
Ljungdahl LG (1986) The autotrophic pathway of acetate synthesis in acetogenic bacteria. Ann. Rev. Microbiol. 40: 415–450
Mackie RI & Bryant MP (1994) Acetogenesis and the rumen: syntrophic relationships. In: Drake HL (Ed.) Acetogenesis (pp. 331–357). Chapman & Hall, New York
Mahler HR & Cordes ER (1971) Biological Chemistry (pp. 520–524). Harper & Row, New York
Melville SB, Michel TA & Macy JM (1988a) Pathway and sites for energy conservation in the metabolism of glucose by Selenomonas ruminantium. J. Bacteriol. 170: 5298–5304
____ (1988b) Regulation of carbon flow in Selenomonas ruminantium grown in glucose-limited continuous culture. J. Bacteriol. 170: 5305–5311
Miller TL (1978) The pathway of formation of acetate and succinate from pyruvate by Bacteroides succinogenes. Arch. Microbiol. 117: 145–1529
Miller TL & Wolin MJ (1973) Formation of hydrogen and formate by Ruminococcus albus. J. Bacteriol. 116: 836–846
Nakajima H, Suzuki K & Imahori K (1978) Purification and properties of acetate kinase from Bacillus stearothermophilus. J. Biochem. 84: 193–203
Nakayama H, Midvinter GG & Krampitz LO (1971) Properties of the pyruvate-formate lyase reaction. Arch. Biochem. Biophys 143: 526–534
Pettipher GL & Latham MJ (1979) Production of enzymes degrading plant cell walls and fermentation of cellobiose by Ruminococcus flavefaciens in batch and continuous culture. J. Gen. Microbiol. 110: 29–38
Pavlostathis SG, Miller TL & Wolin MJ (1988) Fermentation of insoluble cellulose by continuous cultures of Ruminococcus albus. Appl. Environ. Microbiol. 54: 2655–2659
Russell JB & Hino T (1985) Regulation of lactate production in Streptococcus bovis: a spiraling effect that contributes to rumen acidosis. J. Dairy Sci. 68: 1712–1721
Seuber W & Weicher H (1969) Pyruvate carboxylase from Pseudomonas. Methods Enzymol. 13: 259–269
Shi Y & Weimer PJ (1992) Response surface analysis of the effects of pH and dilution rate on Ruminococcus flavefaciens FD-1 in cellulose-fed continuous culture. Appl. Environ. Microbiol. 58: 2583–2591
Smolenski WJ & Robinson JA (1988) In situ rumen hydrogen concentrations in steers fed eight times daily, measured using a mercury reduction detector. FEMS Microbiol. Ecol. 53: 95–100
Stewart CS & Bryant MP (1988) The rumen bacteria. In: Hobson PN (Ed.) The Rumen Microbial Ecosystem (pp. 21–60). Elsevier Applied Science, London
Thauer RK, Jungermann K & Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 41: 100–164
Weimer PJ (1984) Control of product formation during glucose fermentation by Bacillus macerans. J. Gen. Microbiol. 130: 103–111
Weimer PJ, Shi Y & Odt CL (1991) A segmented gas/liquid delivery system for continuous culture of microorganisms on insoluble substrates and its use for growth of Ruminococcus flavefaciens on cellulose. Appl. Microbiol. Biotechnol. 36: 178–183
Wolin MJ (1974) Metabolic interactions among intestinal microorganisms. Am. J. Clin. Nutr. 27: 1320–1328
Wolin MJ & Miller TL (1983) Interactions of microbial populations in cellulose fermentation. Fed. Proc. 42: 109–113
Wood HG, Davis JJ & Willard JM (1969) Phosphenolpyruvate carboxytransphosphorylase from Propionibacterium shermanii. Methods Enzymol. 13: 297–308
Wood WA (1961) Fermentation of carbohydrates and related compounds. In: Gunsalus IC & Stanier RY (Eds) The Bacteria, Vol 2 (pp. 59–149). Academic Press, New York
Zeikus JG, Fuchs G, Kenealy W & Thauer RK (1977) Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J. Bacteriol. 132: 604–613
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shi, Y., Weimer, P. & Ralph, J. Formation of formate and hydrogen, and flux of reducing equivalents and carbon in Ruminococcus flavefaciens Fd-1. Antonie Van Leeuwenhoek 72, 101–109 (1997). https://doi.org/10.1023/A:1000256221938
Issue Date:
DOI: https://doi.org/10.1023/A:1000256221938