Abstract
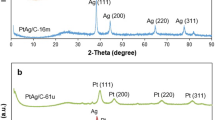
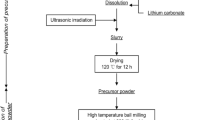
Ball-milling has been used to prepare performing CO tolerant polymer electrolyte fuel cell anode catalysts that contain Pt and Ru. The catalyst precursors are obtained by milling together Pt, Ru and a dispersing agent in the atomic ratio 0.5, 0.5 and 4.0. This precursor is not easily recovered after milling because it sticks to the walls of the vial and on the grinding balls. However, the precursor is recovered as a powder when a process control agent (PCA) is added during the milling step. Various PCAs have been used. The PCA should not interfere with the electrocatalytic activity of the catalysts obtained by leaching the precursor. The best preparation of catalyst precursors are obtained by milling: (i) Pt, Ru and Al (dispersing agent) in the atomic ratio 0.5, 0.5, 4.0 + 10 wt% NaF (PCA) or (ii) Pt , Ru and MgH2 in the 0.5, 0.5, 4.0 atomic or molecular ratio. In this case, MgH2 plays at the same time the role of a dispersing agent and that of a PCA. The catalysts are obtained by leaching Al and NaF in (i) or MgH2 in (ii). The CO tolerance of these catalysts is equivalent to that of Pt0.5Ru0.5 Black from Johnson Matthey. The ball-milled catalysts have a surface area comprised between 30 and 44 m2 g−1. As-prepared catalysts are mainly made of metallic Pt and metallic plus oxidized Ru. After fuel cell tests, Pt is completely metallic while the oxidized Ru content decreases but does not disappear. These catalysts are composed of particles with crystallites of two different sizes: in (i) nanocrystallites (∼4 nm) that contain essentially Pt alloyed with Al and perhaps some Ru, and larger (≥∼30 nm) crystallites that contain essentially Ru; in (ii) Pt nanocrystalline particles that may contain some Ru and larger particles that contain essentially either Ru or Pt.
Similar content being viewed by others
References
S. Gottesfeld and T. A. Zawodzinski, Adv. Electrochem. Sci. Eng. 5 (1997) 195.
P.N. Ross, Jr., In J. Lipkowski and P.N. Ross (Eds), 'Electrocatalysis' (Wiley-VCH, New York, 1998), p. 43.
B.N. Grgur, N.M. Markovic and P.N. Ross, Jr., J. Phys. Chem. B 102 (1998) 2494.
B.N. Grgur, M. Markovic and P.N. Ross, J. Electrochem. Soc. 146 (1999) 1613.
S. Mukerjee, S.J. Lee, E.A. Ticianelli, J. McBreen, B.N. Grgur, N.M. Markovic, P.N. Ross, J.R. Giallombardo and E.S. De Castro, Electrochem. Solid-State Lett. 2 (1999) 12.
L.W. Niedrach, D.W. McKee, J. Paynter and I.F. Danzig, J. Electrochem. Technol. 5 (1967) 318.
D.W. McKee and A. Scarpellino, J. Electrochem. Technol. 6 (1968) 101.
P.N. Ross, K. Kinoshita, A.J. Scarpellino and P. Stonehart, J. Electroanal. Chem. 63 (1975) 97.
A.K. Shukla, P.A. Christensen, A.J. Dickinson and A. Hamnett, J. Power Sources 76 (1998) 54.
A.S. Arico, A.K. Shukla, K.M. El-Khatib, P. Creti and V. Antonucci, J. Appl. Electrochem. 29 (1999) 671.
M. Baldauf and W. Preidel, J. Power Sources 84 (1999) 161.
X. Ren, T. E. Springer and S. Gottesfeld, J. Electrochem. Soc. 147 (2000) 92.
H. Dohle, J. Divisek and R. Jung, J. Power Sources 86 (2000) 469.
X. Ren, P. Zelenay, S. Thomas, J. Davey and S. Gottesfeld, J. Power Sources 86 (2000) 111.
P. Argyropoulos, K. Scott and W.M. Taama, J. Power Sources 87 (2000) 153.
M.P. Hogarth and G. Hards, Platinum Metals Rev. 40 (1996) 150.
A. Hamnett, Catalysis Today 38 (1997) 445.
S. Wasmus and A. Küver, J. Electroanal. Chem. 461 (1999) 14.
M.C. Denis, G. Lalande, D. Guay, J.P. Dodelet and R. Schulz, J. Appl. Electrochem. 29 (1999) 951.
C. Suryanarayana, in C. Suryanarayana (Ed) 'Non-equilibrium Processing of Materials' (Pergamon Materials Series, Pergamon, Amsterdam, 1999) p. 49.
G. Lalande, M.C. Denis, D. Guay, J.P. Dodelet and R. Schulz, J. Alloys Compounds 292 (1999) 301.
B.D. Cullity, in 'Elements of X-ray Diffraction' (Addison-Wesley, 4th edn, 1978).
C. Suryanarayana, in P.W. Lee (Ed), 'Powder Metal Technologies and Applications' (ASM International, Materials Park, OH, 1998), p. 80.
H.A. Gasteiger, P.N. Ross and E.J. Cairns, Surf. Sci. 293 (1993) 67.
J.M. Hutchinson, Plat. Met. Rev. 16 (1972) 88.
J.S. Hammond and N. Winograd, J. Electroanal. Chem. 78 (1977) 55.
J.B. Goodenough, A. Hamnett, B.J. Kennedy, R. Manoharan and S.A. Weeks, J. Electroanal. Chem. 240 (1988) 133.
G.N. Derry and P.N. Ross, Solid State Commun. 52 (1984) 151.
K.A. Daube, M.T. Paffett, S. Gottesfeld and C.T. Campbell, J. Vac. Sci. Technol. A 4 (1986) 1617.
C.D. Wagner, W.W. Riggs, L.E. Davis, J.F. Moulder and G.E. Muilenberg, 'Handbook of X-ray Photoelectron Spectroscopy' (Perkin-Elmer Corporation, 1978).
W.F. Lin, M.S. Zei, M. Eiswirth, G. Ertl, T. Iwasita and W. Vielstich, J. Phys. Chem. B 103 (1999) 6968.
D.R. Rolinson, P.L. Hagans, K.E. Swider and J.W. Long, Langmuir 15 (1999) 774.
J.Y. Shen, A. Adnot and S. Kaliaguine, Appl. Surf. Sci. 51 (1991) 47.
M.T.M. Koper, J.J. Lukkien, A.P.J. Jansen and R.A. van Santen, J. Phys. Chem. B 103 (1999) 5522.
K.A. Friedrich, K.P. Geyzers, A. Marmann, U. Stimming and R.Z. Vogel, Z. Phys. Chem. 208 (1999) 137.
P.K. Shen, K.Y. Chen and A.C.C. Tseung, J. Electrochem. Soc. 142 (1995) L85.
G. Lalande, M.C. Denis, P. Gouerec, D. Guay, J.P. Dodelet and R. Schulz, J. New Mater. Electrochem. Syst. 3 (2000) 185.
F. Delime, J.M. Léger and C. Lamy, J. Appl. Electrochem. 29 (1999) 1249.
J. McBreen and S. Mukerjee, J. Electrochem. Soc. 142 (1995) 3399.
A. G. Gunner, I.T. Hyde, R.J. Potter and D. Thompsett, European Patent Application EP 0838872A2.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Denis, M., Gouérec, P., Guay, D. et al. Improvement of the high energy ball-milling preparation procedure of CO tolerant Pt and Ru containing catalysts for polymer electrolyte fuel cells. Journal of Applied Electrochemistry 30, 1243–1253 (2000). https://doi.org/10.1023/A:1026542821607
Issue Date:
DOI: https://doi.org/10.1023/A:1026542821607