Abstract
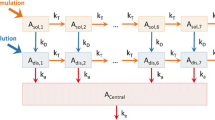
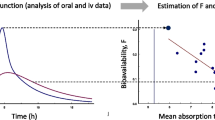
A novel method is described for assessing drug bioavailability from pharmacologic data. The method is based upon a generalized model for the relationship between the observed effect (E) and the input rate (f): E = Φ(ceδ * f), where * denotes convolution, ceδ is effect site unit impulse response (“amount” of drug at the effect site resulting from the instantaneous input of a unit amount of drug) and Φ is transduction function (relates “amount” of drug at the effect site to E). The functions Φ and ceδ are expressed as cubic splines for maximum versatility. Pharmacologic data collected after the administration of two different doses by iv infusion are analyzed simultaneously to estimate the function parameters. This experimental design addresses the fact that Φ and ceδ cannot be uniquely estimated from the results of a single dose experiment. The unknown f from a test treatment is then estimated by applying an implicit deconvolution method to the pharmacologic data collected during that treatment. The method was tested with simulated data. The method and the model were further evaluated by application to a clinical study of verapamil (V) pharmacodynamics in 6 healthy volunteers. Simulations showed that the method is accurate and precise in the presence of a high degree of measurement error, but large intrasubject variability in the model functions can result in biased estimates of the amount absorbed. The method produced reasonably accurate estimates of the V input rate and systemic availability (F) in the 6 human volunteers though there was a trend towards underestimation (estimated total F%=93.6±14 vs. the true F% of 100).
Similar content being viewed by others
REFERENCES
V. F. Smolen. Theoretical and computational basis for drug bioavailability determinations using pharmacological data. I. General consideration and procedure. J. Pharmacokin. Biopharm. 4:337–353 (1976).
V. F. Smolen and R. E. Hannemann. Bioavailability and pharmacologic activity of oral and topical long-acting nitroglycerin: Noninvasive assessment by computerized digital plethysmography. Clin. Res. 27:2–546A (1979).
Department of Health, Education, and Wellfare, FDA. Single-entity coronary vasodilators: Drugs for human use; drug efficacy study implementation; permission for drugs to remain on the market; amendment. Fed. Regist. 42:43127 (1977).
L. B. Sheiner, D. R. Stansky, S. Vozeh, R. D. Miller, and J. Ham. Simultaneous modeling of pharmacokinetics and pharmacodynamics: Application to d-tubocurarine. Clin. Pharmacol. Ther. 25:358–371 (1979).
P. Veng Pedersen and W. R. Gillespie. A system approach to pharmacodynamics I: theoretical framework. J. Pharm. Sci. 77:39–47 (1988).
G. Stagni, A. M. M. Shepherd, Y. Liu, and W. R. Gillespie. New mathematical implementation of generalized pharmacodynamic models: Methods and clinical evaluation. J. Pharmacokin. Biopharm. 25:313–348 (1997).
J. B. Schwartz, D. Verotta, and L. B. Sheiner. Pharmacodynamic modeling of verapamil effects under steady-state and nonsteady-state conditions. J. Pharmacol. Exp. Ther. 251:1032–1038 (1989).
R. E. Kates, D. L. D. Keefe, J. Schwartz, S. Harapat, E. B. Kirsten, and D. C. H. Harrison. Verapamil disposition kinetics in chronic atrial fibrillation. Clin. Pharmacol. Ther. 30:44–51 (1981).
H. Akaike. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 19:716–733 (1974).
S. C. Chow and J. P. Liu. Design and Analysis of Bioavailability and Bioequivalence Studies, Marcel Dekker, New York, 1992.
N. H. G. Holford and T. M. Ludden. Time course of drug effect. In P. G. Wellings and L. P. Balant (eds.), Pharmacokinetics of Drugs, Springer-Verlag, Berlin/New York, 1994, pp. 333–352.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stagni, G., Shepherd, A.M.M., Liu, Y. et al. Bioavailability Assessment from Pharmacologic Data: Method and Clinical Evaluation. J Pharmacokinet Pharmacodyn 25, 349–362 (1997). https://doi.org/10.1023/A:1025775809382
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1025775809382