Abstract
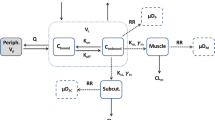
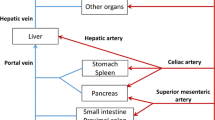
As pan of an overall program to develop a framework for evaluating the contribution of structural and physicochemical properties to pharmacokinetics, the distribution kinetics of nine 5-n-alkyl-5-ethyl barbituric acids in arterial blood and 14 tissues (lung, liver, kidney, stomach, pancreas, spleen, gut, muscle, adipose, skin, bone, heart, brain, testes) was examined after iv bolus administration in rats. The barbituric acids studied form a true homologous series; therefore any differences in pharmacokinetics, noted between congeners, can be directly linked to the increase in lipophilicity, resulting from the addition of a methylene group. A whole-body physiologically based pharmacokinetic model has been developed, assuming most of the tissues to be well-stirred compartments. Brain and testes, in which distribution for the lower homologues was permeability rate-limited, were represented by two compartments. For each homologue, the model parameters have been optimized, using the tissue concentration–time data. The initial distribution processes in the system were very rapid, making it quite stiff, and essentially over before the first samples were taken. A progressively increasing redistribution from lean tissues into adipose on ascending the homologous series was observed, characterized by a tendency for a progressive decrease in the magnitude of the concentration–time profiles for some of the lean and well-perfused tissues, an increase in the adipose concentration–time profile, and an increase in the time to reach the maximum adipose concentration. A shift from permeability rate limitation to perfusion rate limitation of the distribution processes for brain and testes, as well as an increase in the intrinsic hepatic clearance and decrease in the renal clearance with the increase of lipophilicity of the homologues, were quantified. An increase in the total unbound volume of distribution on ascending the homologous series was also observed. Muscle was found to be the major drug depot at steady state, accounting for approximately 50% of the total unbound volume of distribution, regardless of the lipophilicity of the homologue; the unbound volume of distribution of adipose increases more than 10-fold with the increase of lipophilicity.
Similar content being viewed by others
REFERENCES
A. Bernareggi and M. Rowland. Physiological modeling of cyclosporine kinetics in rat and man. J. Pharmacokin. Biopharm. 19:21–50 (1991).
S. Bjorkman, D. R. Stanski, H. Harashima, R. Dowrie, S. R. Harapat, D. R. Wada, and W. F. Ebling. Tissue distribution of fentanyl and alfentanil in the rat cannot be described by a blood flow limited model. J. Pharmacokin. Biopharm. 21:255–279 (1993).
S. Bjorkman, D. R. Wada, D. R. Stanski, and W. F. Ebling. Comparative pharmacokinetics of fentanyl and alfentanil in rats and humans based on parametric single-tissue models. J. Pharmacokin. Biopharm. 22:381–410 (1994).
R. Kawai, M. Lemaire, J.-L. Steimer, A. Bruelisauer, W. Niederberger, and M. Rowland. Physiologically based pharmacokinetic study on a cyclosporine derivative, SDZ IMM 125. J. Pharmacokin. Biopharm. 22:327–365 (1994).
L. E. Gerlovski and R. K. Jain. Physiologically based pharmacokinetic modeling: Principles and applications. J. Pharm. Sci. 72:1103–1129 (1983).
W. F. Ebling, D. R. Wada and D. R. Stanski. From piecewise to full pharmacokinetic modeling: Applied to thiopental disposition in the rat. J. Pharmacokin. Biopharm. 22:259–292 (1994).
S. Toon and M. Rowland. Structure-activity relationships among the barbiturates in the rat. J. Pharmacol. Exp. Ther. 225:752–763 (1983).
Y. Igari, Y. Sugiyama, S. Awazu, and M. Hanano. Comparative physiologically based pharmacokinetics of hexobarbital, phenobarbital and thiopental in the rat. J. Pharmacokin. Biopharm. 10:53–75 (1982).
N. Watari, Y. Sugiyama, N. Kaneniwa, and M. Hiura. Prediction of hepatic first-pass metabolism and plasma levels following intravenous and oral administration of barbiturates in the rabbit based on quantitative structure-pharmacokinetic relationships. J. Pharmacokin. Biopharm. 16:279–301 (1988).
K. Kakemi, T. Arita, R. Hori, and R. Konishi. Absorption and excretion of drugs XXXII. Absorption of barbituric acid derivatives from rat small intestine. Chem. Pharm. Bull. 15:1883–1887 (1967).
T. D. Yih and J. M. Van Rossum. Pharmacokinetics of some homologous series of barbiturates in the intact rat and in the isolated perfused rat liver. J. Pharmacol. Exp. Ther. 203:184–192 (1977).
J. M. Mayer, S. D. Hall, and M. Rowland. Relationship between lipophilicity and tubular reabsorption for a series of 5-alkyl-5-ethyl barbituric acids in the isolated perfused rat kidney preparation. J. Pharm. Sci. 77:359–364 (1988).
R. J. Prankerd and R. Ч. McKeown. Physicochemical properties of barbituric acid derivatives. 2: Partition coefficients of 5, 5-disubstitiuted barbituric acids at 25 C. Int. J. Pharm. 83:25–37 (1992).
R. Pinal and S. H. Yalkowsky. Solubility and partitioning VII: Solubility of barbiturates in water. J. Pharm. Sci. 76:75–85 (1987).
S. H. Steiner, M. J. Moor, and M. H. Bickel. Kinetics of distribution and adipose tissue storage as a function of lipophilicity and chemical structure. 1. Barbiturates. Drug Metab. Dispos. 19:8–14 (1991).
B. B. Brodie, E. Bernstein, and L. C. Mark. The role of body fat in limiting the duration of action of thiopental. J. Pharm. Pharmacol. 105:421–426 (1952).
K. B. Bischoff and R. L. Dedrick. Thiopental pharmacokinetics. J. Pharm. Sci. 57:1346–1351 (1968).
M. Rowland and T. N. Tozer. Clinical Pharmacokinetics: Concepts and Applications, 3rd ed., Lea and Febiger Philadelphia, 1995.
J. M. Gallo, F. C. Lam, and D. G. Perrier. Area method for the estimation of partition coefficients for physiological pharmacokinetic models. J. Pharmacokin. Biopharm. 15:271–280 (1987).
D. Verotta, L. B. Steiner, W. F. Ebling, and D. Stanski. A semiparametric approach to physiological flow models. J. Pharmacokin. Biopharm. 17:463–491 (1989).
B. Davies and T. Morris. Physiological parameters in laboratory animals and humans. Pharm. Res. 10:1093–1095 (1993).
A. C. Heatherington. A physiological based pharmacokinetic model for drug distribution. Ph.D. Thesis, Faculty of Science, University of Manchester, 1994.
R. J. Lutz, R. L. Dedrick, and H. B. Matthews. A preliminary pharmacokinetic model for several biphenyls in the rat. Drug Metab. Dispos. 5:386–396 (1977).
H.-S. G. Chen, and J. F. Gross. Estimation of tissue to plasma partition coefficients used in physiological pharmacokinetic models. J. Pharmacokin. Biopharm. 7:117–125 (1979).
H. Remmer. Induction of drug metabolising enzyme systems in the liver. Eur. J. Clin. Pharmacol. 5:116–136 (1972).
K. C. Skolleborg, J. E. Gronbech, K. Grong, F. E. Abyholm, and J. Lekven. Distribution of cardiac output during pentobarbital versus midazolam/fentanyl/fluanisone anaesthesia in the rat. Lab. Animals 24:221–227 (1990).
M. Gumbleton, P. J. Nicholls, and G. Taylor. Differential influence of laboratory anaesthetic regimens upon renal and hepatosplanchnic haemodynamics in the rat. J. Pharm. Pharmacol. 42:693–697 (1990).
P. C. Kremser and B. L. Gewert. Effect of pentobarbital and haemorrhage on renal autoregulation. Am. J. Physiol. 249:F356–F360 (1985).
W. C. Seyde, L. McGowan, N. Lund, B. Duling, and D. E. Longnecker. Effects of anaesthetics on regional hemodynamics in normovolaemic and hemorrhaged rats. Am. J. Physiol. 249:H164–H173 (1985).
M. J. A. P. Daemen, H. H. W. Thijssen, and H. F. Vervoot-Peters. The effect of pentobarbitone anaesthesia and hypothermia on the hepatic clearance of indocyanine green and S(−)-acenocoumarol in the rat. J. Pharm. Pharmacol. 38:122–125 (1986).
Y. Higashi, N. Notoji, R. Yamajo, and N. Yata. Effect of anaesthesia on drug disposition in the rat. J. Pharmacobiodyn. 5:112–119 (1982).
H. B. Waynforth. Experimental and Surgical Techniques in the Rat, Academic Press, London, 1980, pp. 95–97.
C.-H. Chou, A. M. Evans, G. Fornasini, and M. Rowland. Relationship between lipophilicity and hepatic dispersion and distribution for a homologous series of barbiturates in the isolated perfused in situ rat liver. Drug Metab. Dispos. 21:933–938 (1993).
S. J. Hague, S. M. Nugent, and B. Ford. Computer-based documentation for the NAG library. Lecture Notes Comput. Sci. 142:91–127 (1982).
Author information
Authors and Affiliations
Additional information
An erratum to this article is available at http://dx.doi.org/10.1023/A:1023285010116.
Rights and permissions
About this article
Cite this article
Blakey, G.E., Nestorov, I.A., Arundel, P.A. et al. Quantitative Structure-Pharmacokinetics Relationships: I. Development of a Whole-Body Physiologically Based Model to Characterize Changes in Pharmacokinetics Across a Homologous Series of Barbiturates in the Rat. J Pharmacokinet Pharmacodyn 25, 277–312 (1997). https://doi.org/10.1023/A:1025771608474
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1025771608474