Abstract
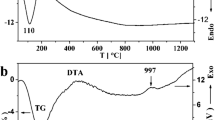
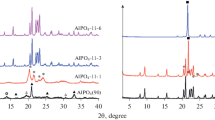
Alkoxy-derived cordierite gels were synthesized from tetraethylorthosilicate (TEOS), aluminum isopropoxide (Al(OPri)3), and magnesium ethoxide (Mg(OEt)2). TEOS was partially hydrolyzed at molar ratios H2O/TEOS = 1.2, in the presence of hydrochloric acid as a catalyst, HCl/TEOS = 0.1. Aluminum and magnesium alkoxides were added successively or as a double alkoxide. Phase transformations occurring in the gel were studied by differential thermal analysis, x-ray diffractometry, and Fourier-transform infrared spectroscopy. In all cases, μ-cordierite crystallized at similar temperatures (950–1000°C) with small amounts of spinel, which confirms dominant influence of the optimal conditions for partial hydrolysis of TEOS on the gels homogeneity. The transformation of μ- into α-cordierite began at about 1100°C. Broadening of diffraction peaks and appearance of new bands in the FT IR spectra confirmed the transformation of α- into modulated β-cordierite at temperatures above 1300°C. Differential thermal analysis under nonisothermal conditions also proved homogeneous nucleation with constant rate and three-dimensional crystallite growth during μ-cordierite crystallization. The overall activation energy of the crystallization of μ-cordierite is 580 ± 81 kJ/mol.
Similar content being viewed by others
References
J.D. Mackenzie, in Ultrastucture Processing of Ceramics, Glasses and Composite, edited by L.L. Hench and D.R. Ulrich (John Wiley &; Sons, New York, 1984), p. 15.
D.R. Uhlmann, B.J.J. Zelinski, and G.E. Wnek, in Better Ceramics Through Chemistry, edited by C.J. Brinker, D.E. Clark, and D.R. Ulrich (MRS Symposia Proceedings, North-Holland, New-York, 1984), Vol. 32, p. 59.
Lj. Kostić-Gvozdenović, S. Milonjić, and R. Ćirjaković, J. Serb. Chem. Soc. 60, 1141 (1995).
Lj. Kostić-Gvozdenović, T. Janaćković, M. Tecilazić-Stevanović, and Dj. Janaćković, in Electroceramics and Ceramics for Special Applications, edited by G. Ziegler and H. Hausner (Proc. II Euro-Ceram. Soc. Conf., Deutsche Keramische Gesellschaft e.V., Frankfurt, 1993), Vol. III, p. 2431.
R. Petrović, Dj. Janaćković, S. Zec, S. Drmanić, and Lj. Kostić-Gvozdenović, J. Mater. Res. 16, 451 (2001).
R. Petrović, Dj. Janaćković, B. Božović, S. Zec, and Lj. Kostić-Gvozdenović, J. Serb. Chem. Soc. 66, 335 (2001).
Dj. Janackovic, V. Jokanovic, Lj. Kostic-Gvozdenovic, S. Zec, and D. Uskokovic, J. Mater. Sci. 32, 163 (1997).
H. Suzuki, K. Ota, and H. Saito, Yogyo-Kyokai-Chi 95, 163 (1987).
K. Maeda, F. Mizukami, S. Miyashita, S. Niwa, and M. Toba, J. Chem. Soc., Chem. Commun. 18, 1268 (1990).
R. Salmon and E. Matijević, Ceram. Int. 16, 157 (1990).
G. Karagedov, A. Feltz, and B. Neidnicht, J. Mater. Sci. 26, 6396 (1991).
M. Okuyama, T. Fukui, and C. Sakurai, J. Am. Ceram. Soc. 75, 153 (1992).
L. El Chahal, J. Werckmann, G. Pourroy, and C. Esnouf, J. Cryst. Grow. 156, 99 (1995).
L. Bonhomme-Coury, F. Babonneau, and J. Livage, Chem. Mater. 5, 323 (1993).
P.N. Kumta, R.E. Hackenberg, P. McMichael, and W.C. Johnson, Mater. Lett. 20, 355 (1994).
D. Pal, A.K. Chakraborty, S. Sen, and S.K. Sen, J. Mat. Sci. 31, 3995 (1996).
K. Langer and W. Schreyer, Am. Mineral. 54, 5442 (1969).
A. Putnis, Contrib. Mineral. Petrol. 74, 135 (1980).
A. Putnis and D.L. Bish, Am. Mineral. 68, 60 (1983).
S.A.T. Redfern, E. Salje, W. Maresch, and W. Schreyer, Am. Mineral. 74, 1293 (1989).
I. Gouby, P. Thomas, D. Mercurio, T. Merle-Méjean, and B. Frit, Mater. Res. Bull. 30, 593 (1995).
C.A. Fyfe, G.C. Gobbi, and A. Putnis, J. Am. Ceram. Soc. 108, 3218 (1986).
B. Güttler, E. Salje, and A. Putnis, Phys. Chem. Minerals 16, 365 (1989).
H. Yinnon and D.R. Uhlmann, J. Non-Cryst. Solids 54, 253 (1983).
K. Matusita and S. Sakka, Phys. Chem. Glasses 20, 81 (1979).
I.W. Donald, J. Mater. Sci. 30, 904 (1995).
D.R. Mc Farlane and M. Fragoulis, Phys. Chem. Glasses 27, 228 (1986).
B.C. Lim and H.M. Jang, J. Mater. Res. 6, 2427 (1991).
H.M. Jang and B.C. Lim, J. Mater. Res. 9, 2627 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Petrović, R., Janaćković, D., Zec, S. et al. Crystallization Behavior of Alkoxy-Derived Cordierite Gels. Journal of Sol-Gel Science and Technology 28, 111–118 (2003). https://doi.org/10.1023/A:1025649406466
Issue Date:
DOI: https://doi.org/10.1023/A:1025649406466