Abstract
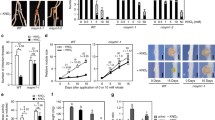
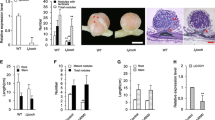
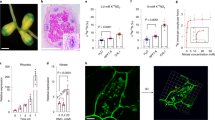
To elucidate the function of the ndx homeobox genes during the Rhizobium-legume symbiosis, two Lotus japonicus ndxgenes were expressed in the antisense orientation under the control of the nodule-expressed promoter Psenod12 in transgenic Lotus japonicus plants. Many of the transformants obtained segregated into plants that failed to sustain proper development and maintenance of root nodules concomitant with down-regulation of the two ndx genes. The root nodules were actively fixing nitrogen 3 weeks after inoculation, but the plants exhibited a stunted growth phenotype. The nodules on such antisense plants had under-developed vasculature and lenticels when grown on medium lacking nitrogen sources. These nodules furthermore entered senescence earlier than the wild-type nodules. Normal plant growth was resumed upon external addition of nitrogen. This suggests that assimilated nitrogen is not properly supplied to the plants in which the two ndx genes are down-regulated. The results presented here, indicate that the ndx genes play a role in the development of structural nodule features, required for proper gas diffusion into the nodule and/or transport of the assimilated nitrogen to the plant.
Similar content being viewed by others
References
Aoyama, T. and Chua, N.H. 1997. A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 11: 605–612.
Appleby C.A. 1984. Leghemoglobin and Rhizobium respiration. Annu. Rev. Plant Physiol. 35: 443–478.
Baima, S., Nobili, F., Sessa, G., Lucchetti, S., Ruberti, I. and Morelli, G. 1995. The expression of the Athb-8 homeobox gene is restricted to provascular cells in Arabidopsis thaliana. Development 121: 4171–4182.
Baima, S., Possenti, M., Matteucci, A., Wisman, E., Altamura, M.M., Ruberti, I. and Morelli, G. 2001. The Arabidopsis ATHB-8 HD-Zip protein acts as a differentiation-promoting transcription factor of the vascular meristems. Plant Physiol. 126: 643–655.
Becker, J., Kemper, E., Schell, J. and Masterson, R. 1992. New plant binary vectors with selectable markers located proximal to the left T-DNA border. Plant Mol. Biol. 20: 1195–1197.
Beeckman, T. and Engler, G. 1994. An easy technique for clearing of histochemically stained plant tissue. Plant Mol. Biol. Rep. 12: 37–42.
Bergersen, F.J. 1982. Root Nodules of Legumes: Structure and Function. Research Studies Press/Wiley, Chichester, UK.
Bladergroen, M.R. and Spaink, H.P. 1998. Genes and signal molecules involved in the rhizobia-Leguminoseae symbiosis. Curr. Opin. Plant Biol. 1: 353–359.
Broughton, W.J. and Dilworth, M. 1971. Control of leghemoglobin synthesis in snake beans. Biochem J. 125: 1075–1080.
Brown, S.M. and Walsh, K.B. 1994. Anatomy of the legume nodule cortex with respect to nodule permeability. Aust. J. Plant. Physiol. 21: 49–68.
Brown, S.M. and Walsh, K.B. 1996. Anatomy of the legume nodule cortex: species survey of suberisation and intercellular glycoprotein. Aust. J. Plant. Physiol. 23: 211–225.
Crespi, M. and Gálvez, S. 2000. Molecular mechanisms in root nodule development. J. Plant Growth Regul. 19: 155–166.
Dalton, A.D., Joyner, S.L., Becana, M., Iturbe-Ormaetxe, I. and Chatfield, J.M. 1998. Antioxidant defenses in the peripheral cell layers of legume root nodules. Plant Physiol. 116: 37–43.
Dénarié, J., Debellé, F. and Promé, J.-C. 1996. Rhizobium lipochitooligosaccharide nodulation factors: signaling molecules mediating recognition and morphogenesis. Annu. Rev. Biochem. 65: 503–535.
Denison, R.F. 1992. Mathematical modeling of oxygen diffusion and respiration in legume root nodules. Plant Physiol. 98: 901–907.
Fenchel, T., King, G.M. and Blackburn, T.H. 1998. Bacterial Biogeochemistry: The Ecophysiology of Mineral Cycling. Academic Press, London.
Foucher, F. and Kondorosi, E. 2000. Cell cycle regulation in the course of nodule organogenesis in Medicago. Plant Mol. Biol. 43: 773–786.
Frazer, H.J. 1942. The occurrence of endodermis in leguminous root nodules and its effect upon nodule function. Proc. R. Soc. Edinb. 61B: 328–343.
Frisch, D.A., Harris-Haller, L.W., Yokubaitis, N.T., Thomas, T.L., Hardin, S.H. and Hall, T.C. 1995. Complete sequence of the binary vector Bin 19. Plant Mol. Biol. 27: 405–409.
Hajdukiewicz, P., Svab, Z. and Máliga, P. 1994. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 25: 989–994.
Handberg, K. and Stougaard, J. 1992. Lotus japonicus, an autogamous, diploid legume species for classical and molecular genetics. Plant J. 2: 487–496.
Hardtke, C.S. and Berleth, T. 1998. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 17: 1405–1411.
Hirsch, A.M. 1992. Developmental biology of legume nodulation. New Phytol. 122: 211–237.
Hiwatashi, Y. and Fukuda, H. 2000. Tissue-specific localization of mRNA for carrot homeobox genes, CHBs, in carrot somatic tissues. Plant Cell Physiol. 41: 639–643.
James, E.K., Sprent, J.I., Minchin, E.R. and Brewin, N.J. 1991. Intercellular location of glycoprotein in soybean nodules: effect of altered rhizosphere oxygen concentration. Plant Cell Environ. 14: 467–476.
Jørgensen, J.E., Grønlund, M., Pallisgaard, N., Larsen, K., Marcker, K.A. and Jensen, E.O. 1999. A new class of plant homeobox genes is expressed in specific regions of determinate symbiotic root nodules. Plant Mol. Biol. 40: 65–77.
Mylona, P., Pawlowsky, K. and Bisseling, T. 1995. Symbiotic nitrogen fixation. Plant Cell 7: 869–885.
Nishitani, C., Demura, T. and Fukuda, H. 2001. Primary phloemspecific expression of a Zinnea elegans homeobox gene. Plant Cell Physiol. 42: 1210–1218.
Pankhurst, C.E. and Sprent, J.I. 1975. Surface features of soybean root nodules. Protoplasma 85: 85–98.
Parsons, R. and Day, D.A. 1990. Mechanism of Soybean nodule adaptation to different oxygen pressures. Plant Cell Environ. 13: 501–512.
Parsons, R., Stanforth, A., Raven, J.A. and Sprent, J.I. 1993. Nodule growth and activity may be regulated by a feedback mechanism involving phloem nitrogen. Plant Cell Environ. 16: 125–136.
Pasquali, G., Goddijn, O.J., de Waal, A., Verpoorte, R., Schilperoort, R.A., Hoge, J.H. and Memelink, J. 1992. Coordinated regulation of two indole alkaloid biosynthetic genes from Catharanthus roseus by auxin and elicitors. Plant Mol. Biol. 18: 1121–1131.
Perret, X., Staehelin, C. and Broughton, W.J. 2000. Molecular Basis of Symbiotic Promiscuity. Microbiology and Molecular Biology Reviews 64: 180–201.
Quaedvlieg, N.E.M., Schlaman, H.R.M., Admiraal, P.C., Wijting, S.E., Stougaard, J. and Spaink, H.P. 1998. Fusions between green fluorescent protein and β-glucuronidase as sensitive and vital bifunctional reporters in plants. Plant Mol. Biol. 37: 715–727.
Ralston, E.J. and Imsande, J. 1982. Entry of oxygen and nitrogen into intact soybean nodules. J. Exp. Bot. 33: 208–214.
Ratcliffe, O.J., Riechmann, J.L. and Zhang, J.Z. 2000. INTERFASCICULAR FIBERLESS1 Is the same gene as REVOLUTA. Plant Cell 12: 315–317.
Scarpella, E., Rueb, S., Boot, K.J.M., Hoge, H.C. and Meijer, A.H. 2000. A role for the rice homeobox gene Oshox1 in provascular cell fate commitment. Development 127: 3655–3669.
Sheng, C. and Harper, J.E. 1997. Shoot versus root signal involvement in nodulation and vegetative growth in wild-type and hypernodulating soybean genotypes. Plant Physiol. 113: 825–831.
Söderman, E., Hjellström, M., Fahleson, J. and Engström, P. 1999. The HD-Zip gene ATHB6 in Arabidopsis is expressed in developing leaves, roots and carpels and up-regulated by water deficit conditions. Plant Mol. Biol. 40: 1073–1083.
Spaink, H.P. 1998. Flavonoids as regulators of plant development: new insights from studies of plant-rhizobia interactions. In: J.T. Romeo, K.R. Downum and R. Verpoorte (Eds.) Phytochemical Signals and Plant-Microbe Interactions. Plenum Press, New York, p. 167–177.
Spaink, H.P. 2000. Root nodulation and infection factors produced by rhizobial bacteria. Annu. Rev. Microbiol. 54: 257–288.
Sprent, J.I. 1980. Root nodule anatomy, type of export product and evolutionary origin in some Leguminosae. Plant Cell Environ. 3: 35–43.
Thykjaer, T., Stiller, J., Handberg, K., Jones, J. and Stougaard, J. 1995. Themaize transposable element Ac is mobile in the legume Lotus japonicus. Plant Mol. Biol. 27: 981–993.
Tornero, P., Conejero, V. and Vera, P. 1996. Phloem-specific expression of a plant homeobox gene during secondary phases of vascular development. Plant J. 9: 639–648.
Van der Fits, L. and Memelink, J. 1997. Comparison of the activities of CaMV 35S and FMV 34S promoter derivatives in atharanthus roseus cells transiently and stably transformed by particle bombardment. Plant Mol. Biol. 33: 943–946.
Vijn, I., Christiansen, H., Lauridsen, P., Kardailsky, I., Quandt, H.-J., Broer, I., Drenth, J., Østergaard Jensen, E., van Kammen, A. and Bisseling, T. 1995. A 200 bp region of the pea ENOD12 promoter is sufficient for nodule-specific and Nod factor induced expression. Plant Mol. Biol. 28: 1103–1110.
Walsh, K. B. 1995. Physiology of the legume nodule and its response to stress. Soil Biol. Biochem. 27: 637–655.
Wycoff, K., Hunt, S., Gonzales, M.B., VandenBosch, K.A., Layzell, D.B. and Hirsch, A.M. 1998. Effects of oxygen on nodule physiology and expression of nodulins in alfalfa. Plant Physiol. 117: 385–395.
Yang, W.-C., de Blank, C., Meskiene, I., Hirt, H., Bakker, J., van Kammen, A., Franssen, H. and Bisseling, T. 1994. Rhizobium Nod factors reactivate the cell cycle during infection and nodule primordium formation, but the cycle is only completed in primordium formation. Plant Cell 6: 1415–1426.
Zhong, R. and Ye, Z.-H. 1999. IFL1, a Gene Regulating Interfascicular Fiber Differentiation in Arabidopsis, Encodes a Homeodomain-Leucine Zipper Protein. Plant Cell 11: 2139–2152.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grønlund, M., Gustafsen, C., Roussis, A. et al. The Lotus japonicus ndxgene family is involved in nodule function and maintenance. Plant Mol Biol 52, 303–316 (2003). https://doi.org/10.1023/A:1023967214199
Issue Date:
DOI: https://doi.org/10.1023/A:1023967214199