Abstract
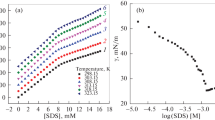
The microphase adsorption–spectral correction (MPASC) technique was applied to the interaction of thioin (TN) with sodium dodecyl sulfate (SDS) at pH 4.56. The synergism mechanism of SDS in solution was analyzed and discussed. The great electrostatic aggregation of TN on SDS obeys Langmuir monolayer adsorption. The property constants of the aggregate were determined and the quantitative determination of the anionic surfactant (AS) in samples was made in the presence of EDTA. Results showed that the large micellar aggregate is (TN–SDS2)31, the adsorption constant of the monomer aggregate is 1.85 × 105 (18°C), and its molar absorptivity is 4.45 × 106 L mol–1 cm–1. For analysis of samples, the recovery is between 94.5 and 111% and the RSD is less than 7.62%.
Similar content being viewed by others
REFERENCE
Ci, Y.X.and Yang, M.M., Chin.Sci.Bull., 1983, vol.16, p.980.
Zheng, Y.X., Li, L.D., and Sun, S.Q., Huaxue Shiji, 1984, vol.6, p.273.
Savvin, S.B., Chernova, R.K., and Kudryavtseva, L.M., Zh.Anal.Khim., 1978, vol.33, p.2127.
Qi, W.B.and Zhu, L.Z., Chem.J.Chin.Univ., 1986, vol.7, p.407.
Zana, R.and Talmon, Y., Nature (London), 1993, vol.362, p.229.
Knaebel, A., Oda, R., Mendes, E., et al., Langmuir, 2000, vol.16, p.2489.
Okano, L.T., Quina, F.H., and El Seoud, O.A., Langmuir, 2000, vol.16, p.3119.
Oda, R., Huc, I., and Candau, S., J.Chem.Commun., 1997, p.2105.
AWWA, J.Am.Water Works Assoc., 1958, p.1343.
Barr, T.et al., J.Soc.Chem.Ind., 1948, vol.67, p.45.
Longwall, J.et al., Analyst (London), 1955, vol.81, p.167.
Fairing, J.D., and Short F.R.Anal.Chem., 1956, vol.28, p.1827.
Gao, H.W.and Ye, Q.S., Can.J.Anal.Sci.Spectrosc., 2000, vol.45, p.154.
Gao, H.W., Quim.Anal., 2001, vol.20, p.153.
Gao, H.W.and Zhou, D.Y., Bull.Korean Chem.Soc., 2002, vol.23, p.29.
Gao, H.W., J.Chin.Chem.Soc. (Taipei), 2002, vol.49, p.33.
Gao, H.W., Jiang, J., and Yu, L.Q., Analyst (Cambridge, UK), 2001, vol.126, p.528.
Gao, H.W., Yang, J.X., Jiang, J., and Yu, L.Q., Supramol.Chem., 2002, vol.14, p.315.
Langmuir, I., J.Am.Chem.Soc., 1918, vol.40, p.1361.
Gao, H.W.and Yu, L.Q., Ann.Chim., 2000, vol.90, p.605.
Gao, H.W., Chen, Y.S., and Li, Y.C., Mikrochim.Acta, 2001, vol.137, p.141.
Gao, H.W.,J.AOAC Int., 2001, vol.84, p.532.
Gao, H.W., Zh.Anal.Khim., 2000, vol.55, p.1065 [J.Anal.Chem.(Engl.Transl.), vol.55, p.958].
Watanabe, H.and Ohmori, H.,Talanta, 1979, vol.26, p.959.
Gao, H.W., Li, Y.C., Zhang, P.F., et al., Zh.Anal.Khim., 2000, vol.55, p.960 [J.Anal.Chem.(Engl.Transl.), vol.55, p. 1007].
Valencia, M.C., Boudra, S., and Bosque-Sendra, M., Analyst (London), 1993, vol.118, p.1333.
Scatchard, G., Scheinerg, I.H., and Armstrong, S.H., J.Am.Chem.Soc., 1950, vol.72, p.535.
Tikhonov, V.N., Zh.Anal.Khim., 1975, vol.30, p.1501.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hong-Wen Gao, Jian-Fu Zhao Langmuir Aggregation of Thionin on Sodium Dodecyl Sulfate and Its Application. Journal of Analytical Chemistry 58, 322–327 (2003). https://doi.org/10.1023/A:1023237412836
Issue Date:
DOI: https://doi.org/10.1023/A:1023237412836