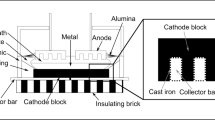
Abstract
Sodium metal can be produced at low temperatures (∼523 K) by electrolysis of sodium tetrachloroaluminate (NaAlCl4) in a cell, which employs sodium ion conducting beta-alumina as diaphragm. A laboratory-scale electrolytic cell and associated systems were designed and constructed to study the various aspects of the energy efficient process. Graphite/reticulated vitreous carbon (RVC) was used as the anode and molten sodium as the cathode. Electrolysis was carried out at ∼523 K with currents in the range 1–10 A (10–125 mA cm−2). The cathodic current efficiency was close to 100%, but the anodic current efficiency was very low (20–30%), probably due to the consumption of chlorine in the intercalation reaction of graphite and aluminium chloride. The sodium metal was analysed by AAS and found to have 5N purity. On prolonged electrolysis, the graphite anode disintegrated due to the formation of ‘graphite intercalation compounds’. RVC behaved as a better chlorine-evolving anode in the initial period of electrolysis, but its ability for chlorine evolution decreased on continuous electrolysis. The study indicated the need for effective stirring of the electrolyte with excess NaCl to avoid build up of aluminium chloride and the resultant complications in the cell.
Similar content being viewed by others

References
M. Sittig, ‘Sodium: Its Manufacture, Properties and Uses’ (Reinhold Publishing Corporation, New York, 1956), pp. 10–44.
A.T. Kuhn, ‘Industrial Electrochemical Processes’ (Elsevier, Netherlands, 1971), p. 99.
Mellor's ‘Comprehensive Treatise on Inorganic and Theoretical Chemistry', Vol. II, Supplement II, The alkali metals, Part I (Longman, 1961), pp. 323–325.
C.H. Lemke, Sodium and its alloys, in ‘Encyclopedia of Chemical Technology', 21 (Kirk Othmer, 1983), p. 193.
British patent 1 200 103 (1967).
British patent 1 155 927 (1969).
British patent 2 193 226 (1988).
K.S. Mohandas, ‘Beta-alumina Based Process for Sodium Manufacture', Report IGC-160 (1994).
Y. Ito, S. Yoshizawa and S. Nakamatsu, J. Appl. Electrochem. 6 (1976) 361.
H. Hayashi, S. Nakamatsu and Y. Ito, J. Appl. Electrochem. 15 (1985) 225.
K.S. Mohandas, PhD thesis, University of Madras, Jan. (2001).
K.S. Mohandas, N. Sanil, Tom Mathews and P. Rodriguez, Metall. Mater. Trans. 32B (2001) 669.
K.S. Mohandas, N. Sanil and P. Rodriguez, in Proceedings of the National Symposium on ‘Electrochemistry in Nuclear Technology’ (Kalpakkam 1998), pp. 157–162.
K.S. Mohandas, N. Sanil, M. Noel and P. Rodriguez, J. Appl. Electrochem. 31 (2001) 997.
H. Wendt, A. Khalil and C.E. Padberg, J. Appl. Electrochem. 21 (1991) 929.
K.S. Mohandas, N. Sanil and P. Rodriguez, in op. cit. [13], pp. 163–168.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohandas, K., Sanil, N. & Rodriguez, P. Design, construction and operation of a laboratory scale electrolytic cell for sodium production using a β″-alumina based low-temperature process. Journal of Applied Electrochemistry 32, 1383–1390 (2002). https://doi.org/10.1023/A:1022644922056
Issue Date:
DOI: https://doi.org/10.1023/A:1022644922056