Abstract
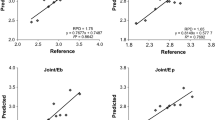
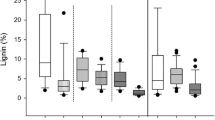
Three commonly employed methods for lignin determination, i.e., the thioglycolic acid (TGA), the acetylbromide (AB), and the acid detergent fiber (ADF) method, were compared using leaves and xylem tissue from five species (Nicotiana tabacum, Populus x canescens, Fagus sylvatica. Quercus robur, and Picea abies). In each case, cell walls were isolated before lignin determination. Each of the three methods estimated a different lignin concentration in a given tissue, except for spruce wood. The “lignin” concentration determined with the AB method was strongly dependent on whether or not the cell walls were subjected to alkaline hydrolysis to remove covalently bound aromatic nonligneous components before lignin determination. Lignin concentrations determined in hydrolyzed cell walls of different tissues and species by the AB method showed a good correlation with those obtained by the TGA method and, thus, were convertible. In contrast, gravimetrically estimated ADF lignins did not or only moderately correlate with lignins measured with methods based on the UV absorbance of the solubilized lignin degradation products. Leaves of a given species generally contained higher ADF-lignin concentrations than the corresponding stem tissue. Both ADF and TGA lignin data of beech were used to calibrate near-infrared reflectance spectra (NIRS) for lignin prediction. Both NIRS calibration procedures gave good statistical fits with correlation coefficients close to 1, indicating that TGA and ADF lignin concentrations of beech can be estimated by NIRS with high accuracy. However, the two calibrations were based on different empirical terms, showing that TGA and ADF lignins did not share the same physical basis for calibration. C/N analysis revealed the presence of 3.1 and 1.4% nitrogen in ADF lignins of beech leaves and wood, respectively. The major fraction of this nitrogen was recovered in amino acids, which corresponded to 14% and 3% protein in ADF lignins of leaves and wood, respectively. These results show that ADF lignins contain significant concentrations of lignin-bound proteins, which renders this method unsuitable to determine genuine lignin.
Similar content being viewed by others
REFERENCES
Akin, D. E., Rigsby, L. L., Gamble, G. R., Morrison III, W. H., Kimball, B. A., Pinter, P. J., Jr., Wall, G. W., Garcia, R. L., and Lamorte, R. L. 1995. Biodegradation of plant cell walls, wall carbohydrates, and wall aromatics in wheat grown in ambient or enriched CO2 concentrations. J. Sci. Food Agric. 67:399–406.
Allen, S. E. 1989. Chemical Analysis of Ecological Materials 2nd ed. Blackwell Scientific Publications, Oxford, England.
AOAC. 1990. Official Methods of Analysis. Association of Official Analytical Chemists, Washington, D.C.
BOLSTER, K., Martin, M., and ABER, J. D. 1996. Determination of carbon fraction and nitrogen concentration in tree foliage by near infrared reflectance: a comparison of statistical methods. Can. J. For. Res. 26:590–600.
Bruce, R. J. and West, C. A. 1989. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol. 91:889–897.
Carpita, N. C. and Gibeaut, D.M. 1993. Structural models of primary cell walls in flowering plants: consistency of the molecular structure with the physical properties of the walls during growth. Plant J. 3:1–30.
Chang, J. Y., Knecht, R., and Braun, D. G. 1992. Amino acid analysis in the picomole range by precolumn derivatization and high-performance liquid chromatography, pp. 41–48, in C.H.W. Hirs, and S. N. Timasheff (eds.). Methods in Enzymology, Enzyme Structure Part 1, Vol. 91, Academic Press, New York.
Dyckmans, J., Flessa, H., Brinkmann, K., Mai, C., and Polle, A. 2002. Carbon and nitrogen dynamics in acid detergent fibre lignins of beech (Fagus sylvatica L.) during the growth phase. Plant Cell Environ. 25:469–478.
Easty, D. B., Berben, S. A., Dethomas, F. A., and Brimmer, P. J. 1990. Near-infrared spectroscopy for the analysis of wood pulp: quantifying hartwood-softwood mixtures and estimating lignin contents. Tappi 257–261.
Fengel, D. and Wegener, G. 1989. Wood. Chemistry, Ultrastructure, Reactions. De Gruyter, Berlin.
Foley, W. J., MCilwee, A., Lawler, I., Aragones, L., Woolnough, A. P., and Berding, N. 1998. Ecological applications of near infra red reflectance spectroscopy—a tool for rapid, cost-effective prediction of the composition of plant animal tissues and aspects of animal performance. Oecology 116:293–308.
Freudenberg, K. 1968. The constitution und biosynthesis of lignin, pp. 47–167, in K. Freudenberg and A. C. Neish (eds.). The Constitution and Biosynthesis of Lignin. Springer Verlag, Heidelberg, Germany.
Gillon, D., Joffre, R., and Dardenne, P. 1993. Predicting the stage of decay of decomposing leaves by near infrared reflectance spectroscopy. Can. J. For. Res. 23:2552–2559.
Gillon, D., Houssard, C., and Joffre, R. 1999. Using near-infrared reflectance spectroscopy to predict carbon, nitrogen and phosphorus content in heterogeneous plant material. Oecology 118:173–182.
Hatfield, R. D., Grabber, J., Ralph, J., and Brei, K. 1999. Using the acetylbromide assay to determine lignin concentrations in herbaceous plants: some cautionary notes. J. Agric. Food Chem. 47:628–632.
Joffre, R., Gillon, D., Agneessens, R., and Biston, R. 1992. The use of near infrared reflectance spectroscopy in litter decomposition studies. Ann. Sci. For. 49:481–488.
Lewis, N. G. and Yamamoto, E. 1990. Lignin: occurence, biogenesis and biodegradation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 41:455–496.
MClellan, T. M., Martin, M., Aber, J. D., Melillo, J. M., Nadelhoffer, K., and Dewey, B. 1991. Comparison of wet chemistry and near infra red reflectance measurements of carbon-fraction chemistry and nitrogen concentration of forest foliage. Can. J. For. Res. 21:1689–1693.
Monties, B. 1989. Lignins. pp. 113–157, in P. M. Dey and J. B. Harborne (eds.). Methods in Plant Biochemistry. Academic Press, New York.
Morrison, I. M. 1972. A semi-micro method for the determination of lignin and its use in prediction the digestibility of forage crops. J. Sci. Food Agric. 23:455–463.
Pillonel, C., Mulder, M. M., Boon, J. J., Forster, B., and Binder, A. 1991. Involvement of cinnamyl-alcohol dehydrogenase in the control of lignin formation in Sorghum bicolor L. Moench. Planta 185:538–544.
Rodrigues, J., Faix, O., and Pereira, H. 1999. Improvement of the acetyl bromide method for lignin determination within large scale screening programmes. Holz Roh-Werkstoff 57:341–345.
Ros Barcelo, A. 1997. Lignification in plant cell walls. Int. Rev. Cytol. 176:87–132.
Rowland, A. P. and Roberts, J. G. 1994. Lignin and cellulose fractionation in decomposition studies using acid-detergent fibre methods. Commun. Soil Sci. Plant Anal. 25:269–277.
Rowland, A. P. and Roberts, J.D. 1999. Evaluation of lignin and lignin.nitrogen fractionation following alternative detergent fibre pre-treatment methods. Commun. Soil Sci. Plant Anal. 30:279–292.
SjÖstrÖm, E. 1993. Wood Chemistry. Fundamentals and Applications. Academic Press, San Diego, California, pp. 80–86.
Strack, D., Heilemann, J., Wray, V., and Dirks, H. 1989. Structures and accumulation patterns of soluble and insoluble phenolics from Norway spruce needles. Phytochemistry 28:2071–2078.
Van Soest, E. 1963. Use of detergents in the analysis of fibrous feeds. II. A rapid method for the determination of fibre and lignin. Assoc. Off. Anal. Chem. J. 46:829–835.
WallbÄcks, L., Edlund, U., Norden, B., and Berglund, I. 1991. Multivariate characterization of pulp using solid-state 13C NMR, FTIR, and NIR. Tappi 201–206.
Wessman, C. A., Aber, J. D., Peterson, D. L., and Melillo, J. M. 1988. Foliar analysis using near infrared spectroscopy. Can. J. For. Res. 18:6–11.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brinkmann, K., Blaschke, L. & Polle, A. Comparison of Different Methods for Lignin Determination as a Basis for Calibration of Near-Infrared Reflectance Spectroscopy and Implications of Lignoproteins. J Chem Ecol 28, 2483–2501 (2002). https://doi.org/10.1023/A:1021484002582
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1021484002582