Abstract
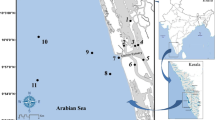
We tested the effects of four different sediment types collected from northern Gulf of Mexico estuarine systems on macroinfaunal colonization and community development in our laboratory flow-through microcosm system. Four sediments, types included , a beach sand, two fine-grained muds, but from different locations, and a 50:50 mixture of one of the mud sediments and beach sand. Our hypothesis was that the pattern of colonization would differ among sediment types based on empirical field data and theory but the differences would be expressed most strongly at sediment type extremes (e.g., mud versus sand). A total of 49 taxa colonized the four sediments. Unidentified Actiniaria (sea anemones) numerically dominated densities among all four sediments with densities ranging between 46.5 to 60.5 per microcosm (20 cm side−1). Average taxa richness per microcosm (N: 10 replicates per sediment treatment) ranged from 10.4 in one of the mud treatments to 14.9 taxa in the sand. These were the only significant differences among sediment types (P≤0.05) in taxa richness and we detected no significant effects of sediment type on animal densities. Differences in community metrics, although statistically significant, were generally of a relatively small magnitude. Five of 10 microcosms per treatment were randomly selected to test for effects of sediment depth (e.g., top, mid, and bottom). In vertically sectioned microcosms, average taxa richness in sand treatments was significantly greater than those of the other three sediments. A non-parametric multivariate analysis (Primer) revealed that community structure in the vertically sectioned microcosms differed significantly between sand and one of the mud treatments. Mean taxa richness of top sections differed significantly from mid and bottom sections. We detected significantly higher animal densities and taxa richness (p≤0.05) in vertically sectioned versus non-sectioned microcosms. However, these differences were unexplained based on experimental protocols.
Similar content being viewed by others
References
Allen, E. J., 1899. On the fauna and bottom-deposits near the thirtyfathom line from the Eddystone to Start Point. J.mar. biol. Assoc. UK 5: 365–542.
Beyers, R. J. & H. T. Odum, 1993. Ecological Microcosms. Springer, New York.
Bonsdorff, E., R. J. Diaz, R. Rosenberg, A. Norkko & G. R. Cutter, Jr., 1996. Characterization of soft-bottom benthic habitats of the Aland Islands, northern Baltic Sea. Mar. Ecol. Prog. Ser. 142: 235–245.
Bray, J. R. & J. T. Curtis. 1957. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 27: 325–349.
Brown, S. S., G. R. Gaston, C. F. Rakocinski & R. F. Heard, 2000. Effects of sediment contaminants and environmental gradients on macrobenthic community trophic structure in the Gulf of Mexico estuaries. Estuaries 23(3): 411–424.
Butman, C. A., 1987. Larval settlement of soft-sediment invertebrates: the spatial scales of pattern explained by active habitat selection and the emerging role of hydrodynamical processes. Oceanogr, Mar. Biol. annu. Rev. 25: 113–165.
Clarke, K. R., 1993. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 18: 117–143.
Clarke, K. R. & R. H. Green, 1988. Statistical design and analysis for a ‘Biological Effects’ study. Mar. Ecol. Prog. Ser. 46: 213–226.
Eckman, J. E., 1983. Hydrodynamic processes affecting benthic recruitment. Limnol. Oceanogr. 28: 241–257.
Flemer, D. A., J. R. Clark, R. S. Stanley, C. M. Bundrick & G. R. Plaia, 1993. The importance of physical scaling factors to benthic marine invertebrate recolonization of laboratory microcosms. Int. J. environ. Stud. 44: 161–179.
Flemer, D. A., R. S. Stanley, B. F. Ruth, C. M. Bundrick, P. H. Moody & J. C. Moore, 1995. Recolonization of estuarine organisms: effects of microcosm size and pesticides. Hydrobiologia 304: 85–101.
Flemer, D. A., B. F. Ruth, C. M. Bundrick & J. C. Moore, 1997. Laboratory effects of microcosm size and the pesticide chlorpyrifos on benthic macroinvertebrate colonization of soft estuarine sediments. Mar. environ. Res. 43(4): 243–263.
Flemer, D. A., W. L. Kruczynski, B. F. Ruth & C. M. Bundrick, 1999. The relative influence of hypoxia, anoxia, and associated environmental factors as determinants of macrobenthic community structure in a Northern Gulf of Mexico estuary. J. aquat. Ecosyst. Stress Recovery 6: 311–328.
Flint, R. W., T. W. Duke & R. D. Kalke, 1982. Benthos investigations: Sediment boxes or natural bottom? Bull. environ. Contam. Toxicol. 28: 257–265.
Gardner, R. H., W.M. Kemp, V. S. Kennedy & J. E. Petersen (Eds.), 2001. Scaling Relations in Experimental Ecology. Columbia University Press, New York.
Gray, J. S., 1974. Animal-sediment relationships. Oceanogr. mar. Biol. ann. Rev. 12: 223–261.
Hansen, D. J. & M. E. Tagatz, 1980. A laboratory test for assessing impacts of substances on developing communities of benthic estuarine organisms. In Eaton, J. G., P. R. Parrish & A. C. Hendricks (eds), Aquatic Toxicology, ASTM STP 707, American Society for Testing and Materials. 40–57.
Highsmith, R. C., 1982. Induced settlement and metamorphosis of sand dollar (Dendraster excentricus) larvae in predator-free sites: adult sand dollar beds. Ecology 63: 329–337.
Karickhoff, S. W., 1981. Semi-empirical estimation of sorption of hydrophobic pollutants on natural sediments and soils. Chemosphere 10: 833–846.
Kemp, W. M., J. E. Petersen & R. H. Gardner, 2001. Scale-dependence and the problem of extrapolation–Implications for experimental and natural coastal ecosystems. In Gardner et al. (eds), Scaling Relations in Experimental Ecology. Columbia University Press, New York. 3–57.
Levin, S., 1992. The problem of pattern and scale in ecology. Ecology 73: 1943-1967.
Levinton, J., 1972. Stability and trophic structure in deposit-feeding and suspension-feeding communities. Am. Nat. 106: 472–486.
Magnuson, J. J., 1990. Long-term ecological research and the invisible present. BioScience 40(7): 495–501.
Marinelli, R. L. & S. A. Woodin, 2002. Experimental evidence for linkages between infaunal recruitment, disturbance, and sediment surface chemistry. Limnol. Oceanogr. 47(1): 221–229.
Neter, J., M. H. Kutner, C. J. Nachtsheim & W. Wasserman, 1996. Applied Linear Statistical Models, 4th edn. Irwin, Inc., Chicago, IL.
Naeem, S., 2001. Experimental validity and ecological scale as criteria for evaluating research programs. In Gardner et al. (eds), Scaling Relations in Experimental Ecology. Columbia University Press, New York. 223–250.
Nowell, A. R. M. & P. A. Jumars, 1987. Flumes: theoretical and experimental considerations for simulation of benthic environments. Oceanogr. mar. Biol. ann. Rev. 25: 91–112.
Olinger, L. W., R. G. Rogers, P. L. Fore, R. L. Todd, B. L. Mullins, F. Bisterfield & L. A. Wise, II, 1975. Environmental and recovery studies of the Escambia Bay and Pensacola Bay system, Florida. U.S. Environmental Protection Agency, Region IV, surveillance and Analysis Division, Escambia Bay Recovery Study, EPA 904/9-76-016, Atlanta, GA.
Pawlik, J. R. & C. A. Butman, 1993. Settlement of a marine tube worm as a function of current velocity: Interacting effects of hydrodynamics and behavior. Limnol. Oceanogr. 38(8): 1730–1740.
Pelegri, S. P. & T. H. Blackburn, 1995. Effect of bioturbation by Nereis sp., Mya arenaria and Cerastoderma sp. on nitrification and denitrification in estuarine sediments. Ophelia 42: 289–299.
Pelegri, S. P., L. P. Nielsen & T.M. Blackburn, 1994. Denitrification in estuarine sediment stimulated by the irrigation activity of the amphipod Corophium volutator. Mar. Ecol. Prog. Ser. 105: 285–290.
Peres, J. M., 1982. In O. Kinne (ed.), Marine Ecology—A Comprehensive Integrated Treatise of Life in Oceans and Coastal Waters, Vol. V, Part I, Chapters 2–9. J. Wiley and Sons, New York.
Petersen, C. G. J., 1918. The sea bottom and its production of fishfood. Rep. Danish Biol. Station 25: 1–62.
Petersen, C. G. J. & P. Boysen-Jensen, 1911. Valuation of the sea. I. Animal life of the sea-bottom, its food and quantity. Rep. Danish Biol. Station 20: 1–76.
Peterson, C. H., 1979. Predation, competition, exclusion, and diversity in soft-sediment benthic communities of estuaries and lagoons. In Livingston, R. J. (ed.), Ecological Processes in Coastal Marine Systems. Plenum, New York. pp. 233–264.
Peterson, C. H., 1982. The importance of predation and intra-and interspecific competition in the population biology of two infaunal suspension-feeding bivalves, Protothaca staminea and Chione undatella. Ecol. Monogr. 52: 437–475.
Peterson, C. H., 1991. Intertidal zonation of invertebrates in sand and mud. Am. Sci. 79: 236–249.
Petersen, J. E., J. C. Cornwell & W.M. Kemp, 1999. Implicit scaling in the design of experimental aquatic ecosystems. Oikos 85: 3–18.
Rhoads, D. C. & D. K. Young, 1970. The influence of deposit feeding benthos on bottom sediment stability and community trophic structure. J. of mar. Res. 28: 150–178.
Ruth, B. F., D. A. Flemer & C. M. Bundrick, 1994. Recolonization of estuarine sediments by macroinvertebrates: Does size matter? Estuaries 17: 606–613.
Sanders, H. L., 1960. Benthic studies in Buzzards Bay. III. The structure of the soft bottom community. Limnol. Oceanogr. 5: 138–153.
SAS, 1989. SAS/STAT User's Guide, Version 6, 4th edn, Volumes 1 and 2, SAS Institute, Inc., Cary, NC.
Snelgrove, P. V. R., 1999. Getting to the bottom of marine biodiversity: sedimentary habitats. BioScience 49(2): 129–138.
Tagatz, M. E., 1986. Some methods for measuring effects of toxicants on laboratory-and field colonized estuarine benthic communities. In Cairns, J. (ed.), Community Toxicity Testing, ASTM STP 920, American Society for Testing and Materials, Philadelphia, PA. 18–29.
Tagatz, M. E. & J. M. Ivey, 1981. Effects of fenvalerate on field and laboratory–developed estuarine benthic communities. Bull. environ. Contam. Toxicol. 27: 256–267.
Tagatz, M. E., J. M. Ivey, N. R. Gregory & J. L. Oglesby, 1981. Effects of pentachlorophenol on field-and laboratory-developed estuarine benthic communities. Bull. environ. Contam. Toxicol. 26: 137–143.
Tagatz, M. E., N. R. Gregory & G. R. Plaia, 1982. Effects of chlorpyrifos on field and laboratory-developed estuarine communities. J. Toxicol. environ. Health 10: 411–421.
Tagatz, M. E., C. H. Deans, J. C. Moore & G. R. Plaia, 1983. Alterations in composition of field-and laboratory-developed estuarine benthic communities exposed to di-n-butyl phthalate. Aquat. Toxicol.3: 239–248.
Tenore, K. R., 1972. Macrobenthos of the Pamlico River estuary, North Carolina. Ecol. Monogr. 42: 51–69.
Thorson, G., 1955. Modern aspects of marine level-bottom animal communities. J. mar. Res. 14: 387–397.
Valiela, I., 1995. Marine Ecological Processes 2nd edn. Springer, New York.
Wiens, J. A., 2001. Understanding the problem of scale in experimental ecology. In Gardner et al. (eds), Scaling Relations in Experimental Ecology. Columbia University Press, New York. 61–88.
Zajac, R. N. & R. B. Whitlatch, 1982. Responses of estuarine infauna to disturbance. I. Spatial and temporal variation of initial recolonization. Mar. Ecol. Prog. Ser. 10: 1–14.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Flemer, D.A., Ruth, B.F. & Bundrick, C.M. Effects of sediment type on macrobenthic infaunal colonization of laboratory microcosms. Hydrobiologia 485, 83–96 (2002). https://doi.org/10.1023/A:1021385901210
Issue Date:
DOI: https://doi.org/10.1023/A:1021385901210