Abstract
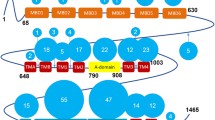
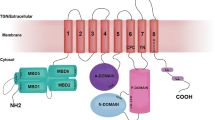
Wilson disease is an autosomal recessive disorder of copper metabolism. The gene for this disorder has been cloned and identified to encode a copper-transporting ATPase (ATP7B), a member of a large family of cation transporters, the P-type ATPases. In addition to the core elements common to all P-type ATPases, the Wilson copper-transporting ATPase has a large cytoplasmic N-terminus comprised six heavy metal associated (HMA) domains, each of which contains the copper-binding sequence motif GMT/HCXXC. Extensive studies addressing the functional, regulatory, and structural aspects of heavy metal transport by heavy metal transporters in general, have offered great insights into copper transport by Wilson copper-transporting ATPase. The findings from these studies have been used together with homology modeling of the Wilson disease copper-transporting ATPases based on the X-ray structure of the sarcoplasmic reticulum (SR) calcium-ATPase, to present a hypothetical model of the mechanism of copper transport by copper-transporting ATPases.
Similar content being viewed by others
REFERENCES
Baker, D., and Sali, A. (2001). Science 294, 93–96.
Beard, S. J., Hashim, R., Membrillo-Hernandez, J., Hughes, M. N., and Poole, R. K. (1997). Mol. Microbiol. 25, 883–891.
Bissig, K. D., Wunderli-Ye, H., Duda, P. W., and Solioz, M. (2001). Biochem. J. 357, 217–223.
Bowmaker, G. A., Clark, G. R., Seadon, J. K., and Dance, I. G. (1984). Polyhedron 3, 535–544.
Bull, P. C., Thomas, G. R., Rommens, J. M., Forbes, J. R., and Cox, D. W. (1993). Nat. Genet. 5, 327–337.
Clark-Baldwin, K., Tierney, D. L., Govindaswamy, N., Gruff, E. S., Kim, C., Berg, J., Koch, S. A., and Penner-Hahn, J. E. (1998). J. Am. Chem. Soc. 120, 8401–8409.
Coucouvanis, D., Murphy, C. N., and Kanodia, S. K. (1980). Inorg. Chem. 19, 2993–2998.
Dance, I. G. (1986). Polyhedron 5, 1037–1104.
Danks, D. M. (1995). In Metabolic Basis of Inherited Disease (Scriver, C. R., Beaudet, A. I., Sly, W. S., and Valle, D., eds.), McGraw-Hill, New York, pp. 2211–2235.
DiDonato, M., Hsu, H. F., Narindrasorasak, S., Que, L., Jr., and Sarkar, B. (2000). Biochemistry 39, 1890–1896.
DiDonato, M., Narindrasorasak, S., Forbes, J. R., Cox, D.W., and Sarkar, B. (1997). J. Biol. Chem. 272, 33279–33282. ATP7B Structure and Function 349
DiDonato, M., Zhang, J., Que, L., Jr., and arkar, B. (2002). J. Biol. Chem. 31, 31.
Fatemi, N., and Sarkar, B. (in press). Inorg. Chim. Acta.
Fiser, A., and Sali, A. (in press). Methods Enzymol.
Forbes, J. R., and Cox, D. W. (1998). Am. J. Hum. Genet. 63, 1663–1674.
Forbes, J. R., and Cox, D. W. (2000). Hum. Mol. Genet. 9, 1927–1935.
Forbes, J. R., Hsi, G., and Cox, D.W. (1999). J. Biol. Chem. 274, 12408–12413.
Fujisawa, K., Imai, S., Kitajima, M., and Moro-oka, Y. (1998). Inorg. Chem. 37, 168–169.
Gitschier, J., Moffat, B., Reilly, D., Wood, W. I., and Fairbrother, W. J. (1998). Nat. Struct. Biol. 5, 47–54]
Goodyer, I. D., Jones, E. E., Monaco, A. P., and Francis, M. J. (1999). Hum. Mol. Genet. 8, 1473–1478.
Guex, N., and Peitsch, M. C. (1997). Electrophoresis 18, 2714–2723.
Hisano, T., Hata, Y., Fujii, T., Liu, J. Q., Kurihara, T., Esaki, N., and Soda, K. (1996). J. Biol. Chem. 271, 20322–20330.
Hou, Z.-J., Narindrasorasak, S., Bhushan, B., Sarkar, B., and Mitra, B. (2001). J. Biol. Chem. 276, 40858–40863.
Jones, D. T., Taylor, W. R., and Thornton, J. M. (1992). Nature 358, 86–89.
Kau, L.-S., Spira-Solomon, D. J., Penner-Hahn, J. E., Hodgson, K. O., and Solomon, E. I. (1987). J. Am. Chem. Soc. 109, 6433–6442.
Koch, S. A., Millar, M., and O'sullivan, T. (1984). Inorg. Chem. 23, 122–124.
Lutsenko, S., Petrukhin, K., Cooper, M. J., Gilliam, C. T., and Kaplan, J. H. (1997). J. Biol. Chem. 272, 18939–18944.
Marti-Renom, M. A., Stuart, A. C., Fiser, A., Sanchez, R., Melo, F., and Sali, A. (2000). Annu. Rev. Biophys. Biomol. Struct. 29, 291–325.
Mense, M., Dunbar, L. A., Blostein, R., and Caplan, M. J. (2000). J. Biol. Chem. 275, 1749–1756.
O'Halloran, T. V. (1993). Science 261, 715–725.
Petris, M. J., Mercer, J. F., Culvenor, J. G., Lockhart, P., Gleeson, P. A., and Camakaris, J. (1996). EMBO J. 15, 6084–6095.
Petrukhin, K., Fischer, S. G., Pirastu, M., Tanzi, R. E., Chernov, I., Devoto, M., Brzustowicz, L. M., Cayanis, E., Vitale, E., Russo, J. J., Matseoane, D., Boukhgalter, B., Wasco, W., Figus, A. L., Loudianos, J., Cao, A., Sternlieb, I., Evgrafov, O., Parano, E., Pavone, L., Warburton, D., Ott, J., Penchaszadeh, G. K., Scheinberg, I. H., and Gilliam, T. C. (1993). Nat. Genet. 5, 338–343.
Pickering, I. J., George, G. N., Dameron, C. T., Kurz, B., Winge, D. R., and Dance, I. G. (1993). J. Am. Chem. Soc. 115, 9498–9505.
Pufahl, R. A., Singer, C. P., Peariso, K. L., Lin, S. J., Schmidt, P. J., Fahrni, C. J., Culotta, V. C., Penner-Hahn, J. E., and O'Halloran, T. V. (1997). Science 278, 853–856.
Ralle, M., Cooper, M. J., Lutsenko, S., and Blackburn, N. J. (1998). J. Am. Chem. Soc. 120, 13525–13526.
Rensing, C., Fan, B., Sharma, R., Mitra, B., and Rosen, B. P. (2000). Proc. Natl. Acad. Sci. U.S.A. 97, 652–656.
Rensing, C., Mitra, B., and Rosen, B. P. (1997). Proc. Natl. Acad. Sci. U.S.A. 94, 14326–14331.
Rensing, C., Sun, Y., Mitra, B., and Rosen, B. P. (1998). J. Biol. Chem. 273, 32614–32617.
Rice, W. J., Young, H. S., Martin, D. W., Sachs, J. R., and Stokes, D. L. (2001). Biophys. J. 80, 2187–2197.
Roberts, E. A., and Cox, D. W. (1998). Baillieres Clin. Gastroenterol. 12, 237–256.
Roelofsen, H., Wolters, H., Van Luyn, M. J., Miura, N., Kuipers, F., and Vonk, R. J. (2000). Gastroenterology 119, 782–793.
Rost, B. (1999). Protein Eng. 12, 85–94.
Sali, A., and Kuriyan, J. (1999). Trends Cell Biol. 9, 20–24.
Sanchez, R., and Sali, A. (1997). Curr. Opin. Struct. Biol. 7, 206–214.
Sanchez, R., and Sali, A. (1998). Proc. Natl. Acad. Sci.U.S.A.95, 13597–13602.
Saqi, M. A., Russell, R. B., and Sternberg, M. J. (1998). Protein Eng. 11, 627–630.
Sass-Kortsak, A. (1975). Pediatr. Clin. North. Am. 22, 963–984.
Scarborough, G. A. (2000). J. Exp. Biol. 203(Pt. 1), 147–154.
Scheinberg, I. H., and Sternlieb, I. (1984). Wilson's Disease, Saunders, Philadelphia, PA.
Schiff, L. A., Nibert, M. L., and Fields, B. N. (1988). Proc. Natl. Acad. Sci. U.S.A. 85, 4195–4199.
Sharma, R., Rensing, C., Rosen, B. P., and Mitra, B. (2000). J. Biol. Chem. 275, 3873–3878.
Silver, S., Nucifora, G., Chu, L., and Misra, T. K. (1989). Trends Biochem. Sci. 14, 76–80.
Sippl, M. J. (1990). J. Mol. Biol. 213, 859–883.
Sippl, M. J. (1993). J. Comput-Aided Mol. Des. 7, 473–501.
Srinivasan, C., Posewitz, M. C., George, G. N., and Winge, D. R. (1998). Biochemistry 37, 7572–7577.
Stokes, D. L., and Green, N. M. (2000). Biophys. J. 78, 1765–1776.
Strausak, D., La Fontaine, S., Hill, J., Firth, S. D., Lockhart, P. J., and Mercer, J. F. (1999). J. Biol. Chem. 274, 11170–11177.
Sweadner, K. J., and Donnet, C. (2001). Biochem. J. 356, 685–704.
Tanzi, R. E., Petrukhin, K., Chernov, I., Pellequer, J. L., Wasco, W., Ross, B., Romano, D. M., Parano, E., Pavone, L., Brzustowicz, L. M., Devoto, M., Peppercorn, J., Bush, A. I., Sternlieb, I., Pirastu, M., Gusella, J. F., Evgrafov, O., Penchaszadeh, G. K., Honig, B., Edelman, I. S., Soares, M. B., Scheinberg, I. H., and Gilliam, T. C. (1993). Nat. Genet. 5, 344–350.
Toyoshima, C., Nakasako, M., Nomura, H., and Ogawa, H. (2000). Nature 405, 647–655.
Tsivkovskii, R., Eisses, J. F., Kaplan, J. H., and Lutsenko, S. (2001a). J. Biol. Chem. 24, 24.
Tsivkovskii, R., MacArthur, B. C., and Lutsenko, S. (2001b). J. Biol. Chem. 276, 2234–2242.
Voskoboinik, I., Greenough, M., La Fontaine, S., Mercer, J. F., and Camakaris, J. (2001). Biochem. Biophys. Res. Commun. 281, 966–970.
Wilson, S. A. K. (1912). Brain 34, 295–508.
Wingfield, P. T., and Pain, R. H. (1996). In Current Protocols in Protein Science (Coligan, J. E., Dunn, B. M., Ploegh, H. L., Speicher, D.W., and Wingfield, P.T., eds.), Wiley, NewYork, Vol. 1, pp. 7.6.1–7.6.23.
Yamaguchi, Y., Heiny, M. E., and Gitlin, J. D. (1993). Biochem. Biophys. Res. Commun. 197, 271–277.
Yamaguchi, Y., Heiny, M. E., Suzuki, M., and Gitlin, J. D. (1996). Proc. Natl. Acad. Sci. U.S.A. 93, 14030–14035.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fatemi, N., Sarkar, B. Structural and Functional Insights of Wilson Disease Copper-Transporting ATPase. J Bioenerg Biomembr 34, 339–349 (2002). https://doi.org/10.1023/A:1021245902195
Issue Date:
DOI: https://doi.org/10.1023/A:1021245902195