Abstract
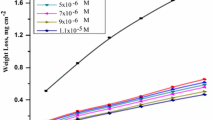
The synergistic effect of iodide ions on the corrosion inhibition of mild steel in 0.5 M sulfuric acid (H2SO4) in the presence of 3,5-bis(4-methylthiophenyl)-4H-1,2,4-triazole (4-MTHT) was investigated using weight loss measurements and different electrochemical techniques such as potentiostatic polarization curves and electrochemical impedance spectroscopy (EIS). The inhibition efficiency (E, %) increased with 4-MTHT concentration, but the desorption potential (E d) remained unchanged with increasing 4-MTHT concentration. The addition of potassium iodide (KI) enhanced E considerably and increased the value of E d. A synergistic effect was observed between KI and 4-MTHT with an optimum mass ratio of [4-MTHT]/[KI] = 4 × 10−2. The synergism parameters (S Θ) calculated from surface coverage were found to be more than unity. This result clearly showed the synergistic influence of iodide ions on the corrosion inhibition of mild steel in 0.5 M H2SO4 by 4-MTHT. The adsorption of this inhibitor alone and in combination with iodide ions followed Langmuir's adsorption isotherm.
Similar content being viewed by others
References
G. Trabanelli, 'Inhibitors for Chemical Cleaning Treatments in Corrosion Inhibitors', Working Party (WP) Report 11 (The Institute of Materials, London, 1994), p. 92.
G. Schmitt, Br. Corros. J. 19 (1984) 165.
G. Schmitt, 'Inhibitors for Chemical Cleaning Treatments in Corrosion Inhibitors', Working Party (WP) Report 11 (The Institute of Materials, London, 1994), p. 64.
P. Chatterejee, M.K. Banerjee and K.P. Mukerjee, Indian J.Technol. 29 (1991) 191.
M.A. Quraishi, J. Rawat and M. Ajmal, Corrosion 55 (1999) 919.
N. Çaliskan and S. Bilgiç, Appl. Surf. Sci. 153 (2000) 128.
E. Stupnisek-Lisac, D. Kasunic and J. Vorkapic-Furac, Corrosion 51 (1995) 767.
Y. Feng, K.S. Siow, W.K. Teo and A.K. Hsieh, Corros. Sci. 41 (1999) 829.
M.M. Al-Abdullah and S.T. Abu-Orabi, Corrosion 22 (1991) 150.
F. Bentiss, M. Lagrenée, M. Traisnel, B. Mernari and H. El Attari, J. Appl. Electrochem. 29 (1999) 1073.
G. Xue and J. Ding, Appl. Surf. Sci. 40 (1990) 327.
G. Xue, J. Ding, P. Lu and J. Dong, J. Phys. Chem. 95 (1991) 7380.
F. Bentiss, M. Lagrenée, M. Traisnel and J.C. Hornez, Corros. Sci. 41 (1999) 781.
M. Lagrenée, B. Mernari, M. Bouanis, M. Traisnel and F. Bentiss, Corros. Sci. 44 (2002) 573.
J.O'M. Bockris and B. Yang, J. Electrochem. Soc. 138 (1991) 2237.
W.J. Lorenz, Z. Phys. Chim. 65 (1970) 244.
J.F. Gueldart and F. Lions, J. Org. Chem. 30 (1965) 318.
F. Bentiss, M. Lagrenée, M. Traisnel and J.C. Hornez, Corrosion 55 (1999) 968.
F. Bentiss, M. Traisnel, L. Gengembre and M. Lagrenée, Appl.Surf. Sci. 152 (1999) 237.
D. Landort, 'Corrosion and Metals Surface Chemistry', (Alden Press, Oxford, 1993), p. 129.
W.J. Lorenz and F. Mansfeld, Corros. Sci. 21 (1981) 647.
J. Heidemeyer and H. Kaesche, Corros. Sci. 8 (1968) 377.
A.A. Aksut, W.J. Lorenz, F. Mansfeld, Corros. Sci. 22 (1982)611.
K.E. Heusler and G.H. Cartledge, J. Electrochem. Soc. 108 (1961)732.
N. Hackerman, E.S. Snavely, Jr. and J.S. Payne, Jr., J. Electrochem.Soc. 113 (1966) 677.
E. McCafferty and N. Hackerman, J. Electrochem. Soc. 119 (1972)146.
L. Elkadi, B. Mernari, M. Traisnel, F. Bentiss and M. Lagrenée, Corros. Sci. 42 (2000) 703.
K. Aramaki and N. Hackerman, J. Electrochem. Soc. 116 (1969)568.
M.A. Quraishi, J. Rawat and M. Ajmal, Corrosion 55 (1999) 919.
Z.A. Iofa, V.V. Batrakov and Cho-Ngok-Ba, Electrochim. Acta 9 (1964) 1645.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bentiss, F., Bouanis, M., Mernari, B. et al. Effect of iodide ions on corrosion inhibition of mild steel by 3,5-bis(4-methylthiophenyl)-4H-1,2,4-triazole in sulfuric acid solution. Journal of Applied Electrochemistry 32, 671–678 (2002). https://doi.org/10.1023/A:1020161332235
Issue Date:
DOI: https://doi.org/10.1023/A:1020161332235