Abstract
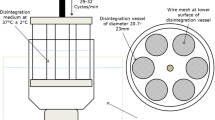
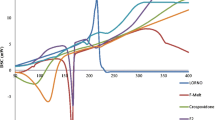
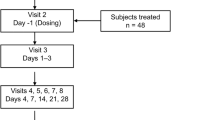
The goal of this study was to examine if the current USP disintegration standard for vitamin C tablets (max. 30 min in water at 37°C with disks) is adequate or if a tighter disintegration standard (e.g., European compendia max. 15 min) should be recommended based on bioavailability considerations. Four formulations of 500-mg vitamin C tablets ranging in mean disintegration time from 9 to 120 min were compared with a standard vitamin C solution in a double-blind clinical trial with 15 subjects. The products were administered with a standard breakfast. The data show that a solution of vitamin C and a fast-disintegrating tablet (8–9 min) have equal but significantly lower bioavailability than tablets with longer disintegration times (30, 60, 120 min). Tablets with a mean disintegration time of 60 min showed the highest bioavailability. When the disintegration test was performed without disks, disintegration times increased so much that only the tablets with the fastest disintegration time (which were also the tablets with the lowest bioavailability) met the current USP disintegration time limit. Based on the results of the study, changes in the USP standard to omit the disks or to shorten the disintegration time will not achieve enhanced bioavailability but will result in reduced vitamin C absorption. In vitro dissolution of vitamin C tablets did not show the traditional relationship with bioavailability.
Similar content being viewed by others
REFERENCES
S. Yung, M. Mayersohn, and B. J. Robinson. Ascorbic acid absorption in man: Influence of divided dose and food. Life Sci. 28:2505–2511 (1981).
S. Yung, M. Mayersohn, and B. J. Robinson. Ascorbic acid absorption in humans: A comparison among several dosage forms. J. Pharm. Sci. 71:282–285 (1982).
G. Zetler, G. Seidel, C. P. Siegers, and H. Iven. Pharmacokinetics of ascorbic acid in man. Europ. J. Clin. Pharmacol. 10:273–282 (1976).
E. Allen. Effect of timed release on the bioavailability of ascorbic acid: Ascorbicaps vs. non timed dosage forms. Curr. Ther. Res. 11:745–749 (1969).
R. Sacharin, T. Taylor, and L. F. Chasseaud. Blood levels and bioavailability of ascorbic acid after administration of a sustained release formulation to humans. Int. J. Vit. Nutr. Res. 47:68–74 (1977).
6.Laboratory Procedures used by the Clinical Chemistry Division, Centers for Disease Control, for the 2nd Health and Nutrition Examination Survey (NHANES II), USDHHS, Public Health Services IV, Analytical Methods, Vitamin C, Atlanta, GA, 1976–1980, pp. 17–19.
G. Levy and W. J. Jusko. Factors affecting the absorption of riboflavin in man. J. Pharm. Sci. 55:285–289 (1966).
M. Mayersohn, Ascorbic acid absorption in man—pharmakokinetic implications. Europ. J. Clin. Pharmacol. 19:140–142 (1972).
Opinion Research Corp. ORC Study 70111, ORC, Princeton, NJ, Mar. 12, 1992.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bhagavan, H.N., Wolkoff, B.I. Correlation Between the Disintegration Time and the Bioavailability of Vitamin C Tablets. Pharm Res 10, 239–242 (1993). https://doi.org/10.1023/A:1018938911420
Issue Date:
DOI: https://doi.org/10.1023/A:1018938911420