Abstract
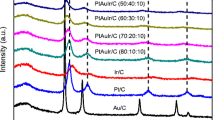
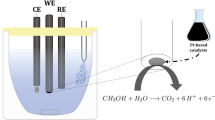
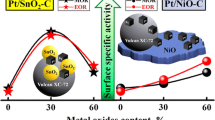
The electrochemical oxidation of methanol was investigated on Pt–Ru–X ternary metallic catalysts (with X=Au, Co, Cu, Fe, Mo, Ni, Sn or W). The catalysts were prepared by electrochemical deposition and dispersed in a conductive three-dimensional matrix, an electronic conducting polymer, polyaniline (PAni). A comparative study of the behaviour of several ternary catalysts towards the electro-oxidation of methanol shows that PAni/Pt–Ru–Mo is the most efficient anode at potentials up to 500mV vs RHE. This latter ternary electrocatalyst leads to current densities up to 10 times higher than those measured with PAni/Pt–Ru in this potential range. Moreover, the catalyst appears to be stable for potentials lower than 550mV vs RHE. According to EDX analysis, the good behaviour of the Pt–Ru–Mo ternary catalyst seems to result from the presence of a small amount of the third metal, at an atomic ratio close to 5%. This set of encouraging results has also been confirmed by preliminary measurements in a single cell direct methanol fuel cell (DMFC) containing a home made PAni/Pt–Ru–Mo anode. The ternary catalyst leads to higher power densities than the PAni/Pt–Ru binary catalyst under the same experimental conditions.
Similar content being viewed by others
References
S. Wasmus and A. Küver, J. Electroanal. Chem. 461 (1999) 14.
C. Lamy and J-M. Léger, Advanced electrode materials for the direct methanol fuel cell, in A. Wieckowski (Ed), `Interfacial Electrochemistry, Theory, Experiments and Applications’, Marcel Dekker, New York, (1999), pp. 885–894.
X. Ren, P. Zelenay, S. Thomas, J. Davey and S. Gottesfeld, J. Power Sources 86 (2000) 111.
K.-A. Adamson and P. Pearson, J. Power Sources 86 (2000) 548.
B.D. McNicol, D.A.J. Rand and K.R. Williams, J. Power Sources 83 (1999) 15.
D. Hart, M.A. Leach, R. Fouquet, P.J. Pearson and A. Bauen, J. Power Sources 86 (2000) 542.
F. Kiso and N. Arashi, Applied Energy 59 (1998) 215.
A. Küver and W. Vielstich, J. Power Sources 74 (1998) 211.
A. Heinzel and V.M. Barragán, J. Power Sources 84 (1999) 70–74.
K. Scott, W.M. Taama, P. Argyropoulos and K. Sundmacher, J. Power Sources 83 (1999) 204.
B. Beden, C. Lamy, A. Bewick and K. Kunimatsu, J. Electroanal. Chem. 121 (1981) 343.
A. Hamnett, Catalysis Today 38 (1997) 445.
A. Hamnett, in A. Wieckowski (Ed), Mechanism of Methanol Electrooxidation in ’Interfacial Electrochemistry, Theory, Experiments and Applications’, Marcel Dekker, New York, (1999), pp. 843–883.
G.T. Burnstein, C.J. Barnett, A.R. Kucernak and K.R. Williams, Catalysis Today 38 (1997) 425.
M.M.P. Janssen and J. Moolhuysen, Electrochim. Acta 21 (1976) 869.
J. Wang, H. Nakajima and H. Kita, J. Electroanal. Chem. 250 (1988) 213.
A. Hamnett, B.J. Kennedy and S.A. Weeks, J. Electroanal. Chem. 240 (1988), 349.
H. Nakajima and H. Kita, Electrochim. Acta 35 (1990) 849.
K. Wang, H.A. Gasteiger, M.N. Markovic and P.N. Ross, Jr., Electrochim. Acta 41 (1996) 2587.
M. Götz and H. Wendt, Electrochim. Acta 43 (1998) 3637.
M. Watanabe, H. Igarashi and T. Fujino, Abstracts of the 192th meeting of the Electrochemical Society and 48th annual ISE meeting, Paris (1997) no. 1083.
S. Mukerjee, S.J. Lee, E.A. Ticianelli and J. McBreen, Abstracts of the 194th meeting of the Electrochemical Society, Boston (1998) no. 1087.
M. Götz and H. Wendt, Abstracts of the 194th meeting of the Electrochemical Society, Boston (1998) no. 1095.
B.N. Grgur, N.M. Markovic and P.N. Ross, Jr., Abstracts of the 194th meeting of the Electrochemical Society, Boston (1998) no. 1097.
T. Zawodzinski, J. Bauman, S. Savett, F. Uribe and S. Gottesfeld, Abstracts of the 194th meeting of the Electrochemical Society, Boston (1998) no. 1100.
S. Mukerjee, S.J. Lee, E.A. Ticianelli, J. McBreen, B.N. Grgur, N.M. Markovic, P.N. Ross, J.R. Giallombardo and E.S. De Castro, Electrochem. Solid-State Lett. 2 (1999) 12.
K. Lasch, L. Jörissen and J. Garche, J. Power Sources 84 (1999) 225–230.
C.T. Hable and M.S. Wrighton, Langmuir 7 (1991) 1305.
C. Lamy, J-M. Léger and F. Garnier, Electrocatalytic properties of conductive polymers in H.S. Nalwa (Ed.), ’Organic Conductive Molecules and Polymers’, Vol. 3 Wiley, Chichester, UK, (1997), p. 471.
S. Swathirajan and Y.M. Mikhail, J. Electrochem. Soc. 139 (1992) 2105.
M. Hepel, J. Electrochem. Soc. 145 (1998) 124.
K.H. Xue, C.X. Cai, H. Yang, Y.M. Zhou, S.G. Sun, S.T. Chen and G. Xu, J. Power Sources 75 (1998) 207.
H. Yang, T. Lu, K. Xue, S. Sun, G. Lu and S. Chen, J. Electrochem. Soc. 144 (1997) 2302.
H. Laborde, J-M. Léger and C. Lamy, J. Appl. Electrochem. 24 (1994) 219.
H. Laborde, J-M. Léger and C. Lamy, J. Appl. Electrochem. 24 (1994) 1019.
W.T. Napporn, H. Laborde, J-M. Léger and C. Lamy, J. Electroanal. Chem. 404 (1996) 153.
W.T. Napporn, J-M. Léger and C. Lamy, J. Electroanal. Chem. 408 (1996) 141.
A.B. Anderson, E. Grantscharova and S. Seong, J. Electrochem. Soc. 143 (1996) 2075.
A.B. Anderson and E. Grantscharova, J. Phys. Chem. 99 (1995) 9143.
P. Shiller and A.B. Anderson, Surf. Sci. 236 (1990) 225.
M. Pourbaix, ’Atlas d’Equilibres Electrochimiques’, Gaultier Villars, Paris, (1963), p. 272.
A. Hamelin, Double-layer properties at sp and sd metal single-crystal electrodes, in B.E. Conway, R.E. White and J.O’M Bockris (Eds), ’Modern Aspects of Electrochemistry’, Vol. 16 (Plenum, New York, 1985), pp. 1–102.
B. Beden, C. Lamy and J-M. Léger, in B.E. Conway, R.E. White and J.O’M Bockris (Eds), ’Modern Aspects of Electrochemistry’, Vol. 22 Plenum, New York, (1992), p. 97.
J.A. Shropshire, J. Electrochem. Soc. 112 (1965) 465.
H. Kita, H. Nakajima and K. Shimazu, J. Electroanal. Chem. 248 (1988) 181.
A. Lima, F. Hahn, J-M. Léger and C. Lamy, in preparation. 386
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lima, A., Coutanceau, C., LÉger, JM. et al. Investigation of Ternary Catalysts for Methanol Electrooxidation. Journal of Applied Electrochemistry 31, 379–386 (2001). https://doi.org/10.1023/A:1017578918569
Issue Date:
DOI: https://doi.org/10.1023/A:1017578918569