Abstract
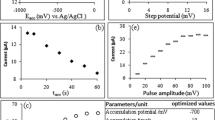
The conditions for the determination of teturam in aqueous–alcoholic solutions by cathodic stripping voltammetry at a silver electrode were found. The detection limit for teturam electroaccumulated for 5 min in a 50% C2H5OH solution alkalized with NaOH to pH 12.7 was 5.6 × 10–5 M (relative standard deviation of 5%) and the upper boundary of analytical range was 5 × 10–4 M (relative standard deviation of 1.5%).
Similar content being viewed by others
REFERENCES
Mashkovskii, M.D., Lekarstvennye sredstva (Medicines), Moscow: Meditsina, 1986, vols. 1, 2, p. 195.
Farmakopeinaya stat'ya (Pharmacopoeia Article) 42–1294–79.
Ivanova, T.E., Pnev, V.V., and Kadorkina, N.A., Inversionnaya vol'tamperometriya dodetsilbenzolsul'fonata natriya na serebryanom electrode (Stripping Voltammetry of Sodium Dodecylbenzenesulfonate on a Silver Electrode), Available from VINITI, 1995, Tyumen', no. 1735.
Zakharova, O.M. and Pnev, V.V., Zh. Anal. Khim., 1999, vol.54, no.10, p.1119.
Weygand, K. and Hilgetag, G., Organisch-Chemische Experimentierkunst (Preparative Organic Chemistry), Barth, J.A., Ed., Leipzig: Verlag, 1964, 3d ed. Translated under the title Metody eksperimenta v organicheskoi khimii, Moscow: Khimiya, 1968.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zakharova, O.M., Zakharov, M.S. Determination of Teturam in Aqueous–Alcoholic Solutions by Cathodic Stripping Voltammetry at a Silver Electrode. Journal of Analytical Chemistry 57, 717–720 (2002). https://doi.org/10.1023/A:1016825909887
Issue Date:
DOI: https://doi.org/10.1023/A:1016825909887