Abstract
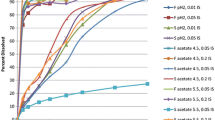
Moexipril {2-[(l-ethoxycarbonyl)-3-phenylpropyl]amino-l-oxopropyl]-6,7-dimethoxy-1,2,3,4-tetra-hydroisoquinoline-3-carboxylic acid (S,S,S)}, an ester prodrug of an ACE inhibitor, was formulated in controlled-release preparations with a range of in vitro release rates, to provide a prolonged input of drug in vivo. However, pharmacokinetic studies with the controlled-release dosage forms in humans produced plasma profiles with the same characteristics and time to peak as an immediate-release capsule. In vitro dissolution data from the controlled-release dosage form, as well as the known characteristics of the polymer used to control drug release from the dosage form, suggest no reason to suspect an abrupt halt to the in vivo release of the drug after 1–2 hr. The lack of sustained blood levels is, therefore, most likely due to failure of the GI tract to absorb the drug beyond some location in the upper small intestine, i.e., site-specific absorption. This theory is supported by a series of computer simulations involving moexipril and the active moiety, moexipril diacid. Possible mechanisms include poor drug permeability, a pH effect whereby the zwitterionic form of the drug is more rapidly absorbed, and esterase cleavage of moexipril to the poorly absorbed moexipril diacid.
Similar content being viewed by others
REFERENCES
J. C. Clinite and H. F. Kabat. J. Am. Pharm. Assoc. 16:74–76 (1976).
A. B. Morrison, C. B. Perusse, and J. A. Campbell. N. Engl. J. Med. 263:115–117 (1960).
E. J. Middleton, E. Nagy, and A. B. Morrison. N. Engl. J. Med. 274:136–139 (1966).
B. N. Swanson, P. A. Veasses, R. K. Ferguson, P. A. Bergquist, and A. E. Till. J. Pharm. Sci. 73:1655–1659 (1984).
K. Lehmann and D. Dreher. Int. J. Pharm. Tech. Prod. Mfr. 2(4):31–43 (1981).
K. Lehmann. Acta Pharm. Fenn. 91:225–238 (1982).
W. B. Abrams, R. O. Davies, and H. J. Gomez. J. Hypertens. 2(Suppl 2):31–36 (1984).
J. B. Dressman. Pharm. Res. 3:123–131 (1986).
W. G. Crouthamel, C. R. Abolin, J. Hsieh, and J. K. Lim. J. Pharm. Sci. 64:1726–1727 (1975).
M. Gibaldi. In Biopharmaceutics and Clinical Pharmacokinetics, Lea and Febiger, Philadelphia, 1977, pp. 15–67.
M. C. Makoid and J. R. Robinson. J. Pharm. Sci. 68:435–443 (1979).
Y. Seta, F. Higuchi, Y. Kawahara, K. Nishimura, and R. Okada. Int. J. Pharm. 41:245–254 (1988).
M. W. Ramsay, G. M. Grass, and J. J. Vallner. Abstract from AAPS Regional Meeting, Reno, Nevada (Feb. 1988).
J. Biollaz, J. L. Schelling, B. Jacot des Combes, D. B. Brunner, G. Desponds, H. R. Brunner, E. H. Ulm, M. Hichens, and H. J. Gomez. Br. J. Clin. Pharm. 14:363–368 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Grass, G.M., Morehead, W.T. Evidence for Site-Specific Absorption of a Novel ACE Inhibitor. Pharm Res 6, 759–765 (1989). https://doi.org/10.1023/A:1015919413103
Issue Date:
DOI: https://doi.org/10.1023/A:1015919413103