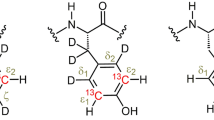
Abstract
31P nuclear spin relaxation measurements have been made on enzyme-bound equilibrium mixtures of lobster-muscle arginine kinase in the presence of substituent activating paramagnetic cation Co(II) (in place of Mg(II)), i.e., on samples in which the reaction, E•CoATP•arginine ⇌ E•CoADP•P-arginine, is in progress. The results have been analyzed on the basis of a previously published theory (Nageswara Rao, B.D. (1995) J. Magn. Reson., B108, 289–293) to determine the structural changes in the reaction complex accompanying phosphoryl transfer. The analysis enables the determination of the change in the Co(II)-31P (γ-P(ATP)) vector as the transferable phosphoryl group moves over and attaches to arginine to form P-arginine. It is shown that the Co(II)-31P distance of ∼3.0 Å, representing direct coordination of Co(II) to γ-P(ATP), changes to ∼4.0 Å when P-arginine is formed in the enzyme-bound reaction complex. This elongation of the Co(II)-31P vector implies an excursion of at least 1.0 Å for the itinerant phosphoryl group on the surface of the enzyme.
Similar content being viewed by others
References
Bernstein, B.E., Michels, P.A.M. and Hol, W.G.J. (1997) Nature, 385, 275–278.
Blethen, S.L. and Kaplan, N.O. (1967) Biochemistry, 6, 1413–1421.
Buttlaire, D.H. and Cohn, M. (1974) J. Biol. Chem., 249, 5733–5740.
Cohn, M. (1959) J. Cell. Comp. Physiol., 54, 17–32.
Gupta, R.K., Fung, C.H. and Mildvan, A.S. (1976) J. Biol. Chem., 251, 2421–2430.
Jarori, G.K., Ray, B.D. and Nageswara Rao, B.D. (1985) Biochemistry, 24, 3487–3494.
Jarori, G.K., Ray, B.D. and Nageswara Rao, B.D. (1989) Biochemistry, 28, 9343–9350.
Leyh, T.S., Goodhart, P.J., Nguyen, A.C., Kenyon, G.L. and Reed, G.H. (1985) Biochemistry, 24, 308–316.
Lin, Y. and Nageswara Rao, B.D. (2000) Biochemistry, 39, 3647–3655.
Mildvan, A.S. and Gupta, R.K. (1978) Meth. Enzymol., 49, 322–359. 21
Morison, J.F. (1973) Enzymes, 8, 457–486.
Nageswara Rao, B.D. (1995) J. Magn. Reson., B108, 289–293.
Nageswara Rao, B.D. (1999a) In NMR in Supramolecular Chemistry, Pons, M. (Ed.), Kluwer Academic Publishers, Dordrecht, pp. 155–170.
Nageswara Rao, B.D. (1999b) Phosphorous Sulfur and Silicon, 144–146, 309–312.
Nageswara Rao, B.D., Buttlaire, D.H. and Cohn, M. (1976) J. Biol. Chem., 251, 6981–6986.
Raghunathan, V., Chau, M.H., Ray, B.D. and Nageswara Rao, B.D. (1999) Biochemistry, 38, 15597–15605.
Ray, B.D. and Nageswara Rao, B.D. (1988) Biochemistry, 27, 5579–5585.
Ray, B.D., Chau, M.H., Fife, W.K., Jarori, G.K. and Nageswara Rao, B.D. (1996) Biochemistry, 35, 7239–7246.
Ray, B.D., Jarori, G.K. and Nageswara Rao, B.D. (1999) J. Magn. Reson., 136, 130–133.
Ray, B.D., Rösch, P. and Nageswara Rao, B.D. (1988) Biochemistry, 27, 8669–8676.
Schulz, G.Z., Müller, C.W. and Diederichs, K. (1990) J. Mol. Biol., 213, 627–630.
Vasavada, K.V., Kaplan, J.I. and Nageswara Rao, B.D. (1980) J. Magn. Reson., 41, 467–482.
Villafranca, J.J. (1984) In Phosphorous-31 NMR: Principles and Applications, Gorenstein, D.G. (Ed.), Academic Press, New York, NY, pp. 155–174.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ray, B.D., Jarori, G.K. & Nageswara Rao, B. Quantitation of movement of the phosphoryl group during catalytic transfer in the arginine kinase reaction: 31P relaxation measurements on enzyme-bound equilibrium mixtures** . J Biomol NMR 23, 13–21 (2002). https://doi.org/10.1023/A:1015313521182
Issue Date:
DOI: https://doi.org/10.1023/A:1015313521182