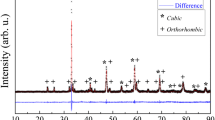
Abstract
This paper presents an investigation of the performance and stability for oxygen reduction on in situ oxidized Ni alloys, specially focused on 95 at % Ni + 5 at % Al and 85 at % Ni + 15 at % Al alloy electrodes in Li/Na carbonate eutectic. Test specimens of the alloys were prepared as thin film electrodes sputtered onto Au substrates. In situ oxidation of alloy electrodes and electrochemical measurements for oxygen reduction on the electrodes were performed in the free-volume melt at 923 K. It was found that the in situ oxidized Ni + Al alloys exhibit higher performance for the oxygen reduction than the NiO without Al. Electrochemical fractal analysis (EFA) revealed higher oxide film stability of the Ni + Al alloys in comparison to NiO electrodes. The surface morphology of the alloy specimen after oxidation was investigated with SEM and AFM.
Similar content being viewed by others
References
T. Fukui, H. Okawa and T. Tsunooka, J. Power Sources 71 (1998) 239.
I. Uchida, Y. Mugikura, T. Nishina and K. Itaya, J. Electroanal. Chem. 206 (1986) 241.
T. Nishina, K. Takizawa and I. Uchida, J. Electroanal. Chem. 263 (1989) 87.
K. Yamada and I. Uchida, Chem. Lett. (1994) 299.
K. Yamada and I. Uchida, J. Electroanal. Chem. 385 (1995) 57.
P. Tomczyk, H. Sato, K. Yamada, T. Nishina and I. Uchida, J. Electroanal. Chem. 391 (1995) 125.
P. Tomczyk, H. Sato, K. Yamada, T. Nishina and I. Uchida, J. Electroanal. Chem. 391 (1995) 133.
C.G. Lee, K. Yamada, T. Nishina and I. Uchida, J. Power Sources 62 (1996) 145.
K. Yamada, N. Sato, T. Fujino, M. Nishizawa and I. Uchida, Electrochem. 67 (1999) 68.
M. Mohamedi, Y. Hisamitsu, T. Kudo, T. Itoh, and I. Uchida, J. Solid State Electrochem. 5 (2001) 538.
T. Kudo, Y. Hisamitsu, K. Kihara, M. Mohamedi and I. Uchida, J. Power Sources 104 (2002) 272.
R.B. Swaroop, J.W. Sim and K. Kinoshita, J. Electrochem. Soc. 125 (1978) 1799.
N. Fujimoto, M. Yamamoto and T. Nagoya, J. Power Sources 71 (1998) 231.
T. Ohzuku, A. Ueda and M. Kouguchi, J. Electrochem. Soc. 142 (1995) 4033.
H.M. Hindam and W.W. Smeltzer, J. Electrochem. Soc. 127 (1980) 1622.
C.T. Liu and O.F. Devereux, J. Electrochem. Soc. 138 (1991) 3349.
R.D. Shannon, Acta Cryst. A32 (1976) 751.
T. Pajkossy, J. Electroanal. Chem. 300 (1991) 1.
P. Ocon, P. Herrasti, L. Vazquez, R.C. Salvarezza, J.M. Vara and A. J. Arvia, J. Electroanal. Chem. 319 (1991) 101.
T. Nishina, I. Uchida and J.R. Selman, J. Electrochem. Soc. 141 (1994) 1191.
M.S. Mattsson, G.A. Niklasson and C.G. Granqvist, Phys. Rev. B 54 (1996) 17 884.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kudo, T., Hisamitsu, Y., Kihara, K. et al. Electrochemical behaviour of Ni + Al alloy as an alternative material for molten carbonate fuel cell cathodes. Journal of Applied Electrochemistry 32, 179–184 (2002). https://doi.org/10.1023/A:1014790013730
Issue Date:
DOI: https://doi.org/10.1023/A:1014790013730