Abstract
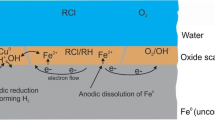
Oxidation reduction potential (ORP) changes were monitored during the course of the remediation of four wastewater matrices by metallic iron (Fe0) based on a batch fluidized bed reactor. Two of these matrices contained azo dyes (Acid Orange II and Acid Blue 113), another contained pentachlorophenol and the fourth was an authentic dyewaste. For the azo dye Acid Orange II ORP was found to follow the same trend as the dye concentration ([AOII]), decreasing exponentially with time over the course of the remediation. Change in ORP was found to be directly proportional to [AOII] and to follow a logarithmic relationship with [Fe2+]2[aa]2[AOII]−1, indicating a Nernstian behaviour. It is concluded that the ratio of remediation products to reactants can be determined directly by monitoring changes in ORP. The electrochemical conditions that influence corrosion were found to control remediation, consistent with the remediation being driven by anaerobic corrosion and predicted from potential–pH Pourbaix diagrams.
Similar content being viewed by others
References
P. Bowden, Waste and Water Treatment 32 (1989) 21.
A. Ghauch, Chemsosphere 43 (2001) 1109.
S. Choe, S-H. Lee, Y-Y. Chang, K-Y. Hwang and J. Khim, Chemosphere 42 (2001) 367.
A. Ghauch, C. Gallet, A. Charef, J. Rima and M. Martin-Bouyer, Chemosphere 42 (2001) 419.
T. Dombek, E. Dolan, J. Schultz and D. Klarup, Environmental Pollution 111 (2001) 21.
A. Ghauch and J. Suptil, Chemosphere 41 (2000) 1835.
W. Feng, D. Nansheng and H. Helin, Chemosphere 41 (2000) 1233.
A.R. Gavaskar, B.M. Sass, E. Drescher, L. Cumming, D. Giammar and N. Gupta, First International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Vol. 1 (1998), p. 91.
R. Muftikian, Q. Fernando and N. Korte, Wat. Res. 29 (1995) 2434.
F.L. Laque and H.R. Copson, ‘Corrosion Resistance of Metals and Alloys’ (Reinhold, New York, 2nd edn, 1963).
T. Bigg and S.J. Judd, Environ. Tech. 21 (2000) 661.
T. Bigg and S.J. Judd, Trans. I Chem E Part B, 79 (2001) 297.
P.G. Tratnyek, Chemistry and Industry, July (1996) 409.
J.C. Scully, ‘The Fundamentals of Corrosion’ (Pergamon, Oxford, 3rd edn, 1990).
E.J. Weber and N.L. Wolfe, Environ. Toxicol. Chem. 6 (1987) 911.
C-P.C. Yen, T.A. Perenich and G.L. Baughman, Environ. Toxicol. Chem. 10 (1991) 109.
E.J. Weber and R.L. Adams, Environ. Sci. Technol. 29 (1995) 1163.
J.M. Smolen, E.J. Weber and P.G. Tratnyek, Environ. Sci. Technol. 33 (1999) 440.
J. Cao, W. Liping, Q. Huang, L. Wang and S. Han, Chemosphere 38 (1999) 565.
S. Nam and P.G. Tratnyek, Wat. Res. 34 (2000) 1837.
R.W. Gillham and S.F. O'Hannesin, Ground Water 32 (1994) 958.
D.W. Blowes, C.J. Ptacek and J.L. Jambor, Environ. Sci. Technol. 31 (1997) 3348.
D.P. Siantar, C.G. Schreier, C. Chou and M. Reinhard, Wat. Res. 30 (1996) 2315.
L.L. Shreir, ‘Corrosion, 1 Metal/Environment Reactions’ (Newnes-Butterworths, London, 1976).
T. Boronina, K. Klabunde and G. Segeev, Environ. Sci. Technol. 29 (1995) 1511.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bigg, T., Judd, S. Electrochemical monitoring of water remediation by metallic iron. Journal of Applied Electrochemistry 31, 1339–1344 (2001). https://doi.org/10.1023/A:1013833717336
Issue Date:
DOI: https://doi.org/10.1023/A:1013833717336