Abstract
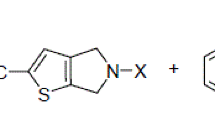
High selectivity has been discovered for the C-alkylation of ambident anions of 5-oxo-1H-4,5-dihydroindeno[1,2-b]pyridines with ethyl bromoacetate, allyl, propargyl, and phenacyl bromides, which leads to the formation of the corresponding 4a-substituted 5-oxo-4H-4a,5-dihydroindeno[1,2-b]pyridines in high yield.
Similar content being viewed by others
REFERENCES
V. K. Lusis, D. Kh. Mutsenietse, A. Z. Zandersons, I. B. Mazheika, and G. Ya. Dubur, Khim.Geterotsikl.Soedin., 393 (1984).
V. Lusis, D. Muceniece, and G. Duburs, Tetrahedron, 42, 1548 (1986).
I. Petrov, I. Saper, and B. Sturgeon, J.Chem.Soc., 2134 (1949).
E. Ya. Ozola and G. Ya. Vanag, Khim.Geterotsikl.Soedin., 103 (1969).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Muceniece, D., Stupnikova, S. & Lusis, V. 4a-Alkyl Derivatives of 5-Oxo-4H-4a,5-dihydroindeno[1,2-b]pyridine. Chemistry of Heterocyclic Compounds 37, 981–986 (2001). https://doi.org/10.1023/A:1012787517710
Issue Date:
DOI: https://doi.org/10.1023/A:1012787517710