Abstract
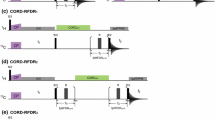
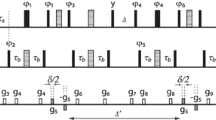
Experiments are proposed for the measurement of the vicinal coupling constants between β-carbons and either amide protons of the same or carbonyl carbons of the preceding amino acid residue in 13C/15N-labeled proteins. Both couplings depend on the backbone torsional angle φ. The three-dimensional pulse sequences give rise to E.COSY-like multiplet patterns in which heteronuclear one-bond couplings separate the doublet components corresponding to the two spin states of the respective passive nuclei. Thus, in contrast to previously published pulse schemes which employed the homonuclear 1J(Cα,Cβ) interaction, difficulties due to overlap of spectral regions of active and passive spins are avoided. A major drawback of the novel sequences is their limited sensitivity. Nevertheless, application to Desulfovibrio vulgaris flavodoxin yielded coupling constants for more than 85% of all non-glycine and non-proline residues.
Similar content being viewed by others
References
Andersson, P., Gsell, B., Wipf, B., Senn, H. and Otting, G. (1998) J. Biomol. NMR, 11, 279–288.
Archer, S.J., Ikura, M., Torchia, D.A. and Bax, A. (1991) J. Magn. Reson., 95, 636–641.
Blümel, M., Schmidt, J.M., Löhr, F. and Rüterjans, H. (1998) Eur. Biophys. J., 27, 321–334.
Brüschweiler, R. and Case, D.A. (1994) J. Am. Chem. Soc., 116, 11199–11200.
Bystrov, V.F. (1976) Prog. NMR Spectrosc., 10, 41–81.
Cowburn, D., Live, D.H., Fischman, A.J. and Agosta, W.C. (1983) J. Am. Chem. Soc., 105, 7435–7442.
Delaglio, F., Torchia, D.A. and Bax, A. (1991) J. Biomol. NMR, 1, 439–446.
Emsley, L. and Bodenhausen, G. (1990) Chem. Phys. Lett., 165, 469–476.
Geen, H. and Freeman, R. (1991) J. Magn. Reson., 93, 93–141.
Goldman, M. (1984) J. Magn. Reson., 60, 437–452.
Griesinger, C., Sørensen, O.W. and Ernst, R.R. (1985) J. Am. Chem. Soc., 107, 6394–6396.
Griesinger, C., Sørensen, O.W. and Ernst, R.R. (1986) J. Chem. Phys., 85, 6837–6852.
Griesinger, C., Sørensen, O.W. and Ernst, R.R. (1987) J. Magn. Reson., 75, 474–492.
Grzesiek, S. and Bax, A. (1993) J. Am. Chem. Soc., 115, 12593–12594.
Hoch, J.C., Dobson, C.M. and Karplus, M. (1985) Biochemistry, 24, 3831–3841.
Hu, S.-J. and Bax, A. (1997) J. Am. Chem. Soc., 119, 6360–6368.
Hu, S.-J. and Bax, A. (1998) J. Biomol. NMR, 11, 199–203.
Jahnke, W., Baur, M., Gemmecker, G. and Kessler, H. (1995) J. Magn. Reson., B106, 86–88.
Karplus, M. (1959) J. Chem. Phys., 30, 11–15.
Karplus, M. (1963) J. Am. Chem. Soc., 85, 2870–2871.
Kay, L.E., Keifer, P. and Saarinen, T. (1992) J. Am. Chem. Soc., 114, 10663–10665.
Kay, L.E., Xu, G.Y. and Yamazaki, T. (1994) J. Magn. Reson., A109, 129–133.
Knauf, M., Löhr, F., Blümel, M., Mayhew, S.G. and Rüterjans, H. (1996) Eur. J. Biochem., 238, 423–434.
Löhr, F. and Rüterjans, H. (1995) J. Biomol. NMR, 5, 25–36.
Löhr, F., Blümel, M., Schmidt, J.M. and Rüterjans, H. (1997) J. Biomol. NMR, 10, 107–118.
Madsen, J.C. and Sørensen, O.W. (1992) J. Magn. Reson., 100, 431–436.
Madsen, J.C., Sørensen, O.W., Sørensen, P. and Poulsen, F.M. (1993) J. Biomol. NMR, 3, 239–244.
Marion, D. and Wüthrich, K. (1983) Biochem. Biophys. Res. Commun., 113, 967–974.
Marion, D., Ikura, M., Tschudin, R. and Bax, A. (1989a) J. Magn. Reson., 85, 393–399.
Marion, D., Ikura, M. and Bax, A. (1989b) J. Magn. Reson., 84, 425–430.
Matsuo, H., Kupčce, Ē., Li, H. and Wagner, G. (1996) J. Magn. Reson., B111, 194–198.
Meissner, A., Duus, J.Ø. and Sørensen, O.W. (1997a) J. Biomol. NMR, 10, 89–94.
Meissner, A., Duus, J.Ø. and Sørensen, O.W. (1997b) J. Magn. Reson., 128, 92–97.
Meissner, A., Schulte-Herbrüggen, T. and Sørensen, O.W. (1998a) J. Am. Chem. Soc., 120, 3803–3804.
Meissner, A., Schulte-Herbrüggen, T. and Sørensen, O.W. (1998b) J. Am. Chem. Soc., 120, 7989–7990.
Palmer III, A.G., Cavanagh, J., Wright, P.E. and Rance, M. (1991) J. Magn. Reson., 93, 151–170.
Schmidt, J.M., Ernst, R.R., Aimoto, S. and Kainosho, M. (1995) J. Biomol. NMR, 6, 95–105.
Schmidt, J.M., Blümel, M., Löhr, F. and Rüterjans, H. (1999) J. Biomol. NMR, in press.
Schmieder, P. and Kessler, H. (1992) Biopolymers, 32, 435–440.
Seip, S., Balbach, J. and Kessler, H. (1994) J. Magn. Reson., B104, 172–179.
Shaka, A.J., Barker, P.B. and Freeman, R. (1985) J. Magn. Reson., 64, 547–552.
Shaka, A.J., Lee, C.J. and Pines, A. (1988) J. Magn. Reson., 77, 274–293.
Stockman, B.J., Richardson, T.E. and Swenson, R.P. (1994) Biochemistry, 33, 15298–15308.
Stonehouse, J., Shaw, G.L., Keeler, J. and Laue, E.D. (1994) J. Magn. Reson., A107, 178–184.
Tjandra, N., Szabo, A. and Bax, A. (1996) J. Am. Chem. Soc., 118, 6986–6991.
Wagner, G. (1990) Prog. NMR Spectrosc., 22, 101–139.
Wang, A.C. and Bax, A. (1995) J. Am. Chem. Soc., 117, 1810–1813.
Wang, A.C. and Bax, A. (1996) J. Am. Chem. Soc., 118, 2483–2494.
Watt W., Tulinsky, A., Swenson, R.P. and Watenpaugh, K.D. (1991) J. Mol. Biol., 218, 195–208.
Wittekind, M. and Mueller, L. (1993) J. Magn. Reson., B101, 201–205.
Zhu, G. and Bax, A. (1990) J. Magn. Reson., 90, 405–410.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Löhr, F., Rüterjans, H. Alternative E.COSY techniques for the measurement of 3J(C ′i −1,C βi ) and 3 J(H Ni ,C βi ) coupling constants in proteins. J Biomol NMR 13, 263–274 (1999). https://doi.org/10.1023/A:1008378719908
Issue Date:
DOI: https://doi.org/10.1023/A:1008378719908