Abstract
Purpose. Two in vitro test systems used to study drug penetration into human skin—the Franz diffusion cell (FD-C) and the Saarbruecken penetration model (SB-M)—were evaluated, and the results were compared with data gained under analogous in vivo conditions.
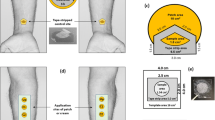
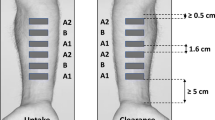
Methods. Excised human skin was used in all in vitro experiments. Flufenamic acid dissolved in wool alcohols ointment, was chosen as a model drug, and the preparation was applied using ‘infinite dose’ conditions. To acquire quantitative information about the drug penetration, the skin was segmented into surface parallel sections at the end of each experiment, first by tape stripping the stratum corneum (SC), and second by cutting the deeper skin layers with a cryomicrotome. The flufenamic acid was extracted from each sample and assayed by high performance liquid chromatography (HPLC). For in vivo experiments, only the tape stripping technique was used.
Results. a) Drug penetration into the SC: In both in vitro test systems the total drug amounts detected in the SC were found to increase over the different incubation times. Similar conditions were obtained in vivo, but on a lower level. Using Michaelis-Menten kinetics, the mmax value was calculated for the skin of two donors. The relations of the mmax values for the FD-C and the SB-M closely correspond (1.26 [donor 1] and 1.29 [donor 2]). A direct linear correlation of the drug amount in the SC and the time data were found for in vivo with both in vitro test systems.%b) Drug penetration into the deeper skin layers: The detected drug amounts in the deeper skin layers continuously increased with the incubation time in the SB-M, while in the FD-C, only very small drug amounts were observed after incubation times of 30 and 60 minutes. It was also noticed, that the drug amounts rose steeply at time points 3 and 6 hours. Additional studies showed a remarkable penetration of water into the skin from the basolateral acceptor compartment in the FD-C. This could explain the different drug transport into the deeper skin layers between the two in vitro test systems.
Conclusions. Both in vitro models showed comparable results for the drug penetration into the SC and a robust correlation with in vitro data. Different results were obtained for the deeper skin layers. Whether a correlation between in vitro and in vivo data is also possible here has to be investigated by further experiments.
Similar content being viewed by others
REFERENCES
S. A. Akhter and B. W. Barry. Permeation of drugs through human skin: method and design of diffusion cells for in vitro use. In: R. Marks and G. Plewig, Skin models, Springer Verlag, New York, 1986 p. 358–370.
R. L. Bronaugh. Diffusion cell design. In: V. P. Shah and H. I. Maibach, Topical drug bioavailability, bioequivalence and penetration, Plenum Press, New York/London, 1993, pp. 117–125.
T. J. Franz. Percutaneous absorption—on the relevance of in vitro data. J. Invest. Dermatol. 64:190–195 (1975).
M. Hailer. Freisetzung von Salicylsaeure aus Suspensionssalben: Liberation im Membranmodell im Vergleich zur Wirkstoffabgabe an excidierter Haut. Dissertation Saarbruecken 1981.
S. Blasius. Sorptionsvermittler—Einfluss auf die Liberation von Indomethacin aus Salben and auf die Arzneistoffaufnahme durch excidierte Haut. Dissertation Saarbruecken 1985.
D. Borchert. Methoden zur Untersuchung der simultanen Penetration von Arzneistoffen and Vehikelbestandteilen aus Salben in exzidierter Humanhaut. Dissertation Saarbrücken 1994.
T. J. Franz, P. A. Lehman, S. F. Franz, H. North-Root, J. L. Demetrulias, C. K. Kelling, S. J. Moloney, and S. D. Gettings. Percutaneous penetration of N-nitrosodiethanolamine through human skin (in vitro): Comparison of finite and infinite dose applications from cosmetic vehicles. Fundam. Applied Toxiccol. 21:213–221 (1993).
T. Wild. Einfluss der physikochemischen Eigenschaften von Arzneistoffen and Vehikeln auf die Permeabilitaet der menschlichen Hornschicht. Dissertation Saarbruecken 1988.
F. Theobald. In-vitro Methoden zur biopharmazeutischen Qualitaetspruefung von Dermatika unter Beruecksichtigung der Lipidzusammensetzung des Stratum corneum. Dissertation Saarbruecken 1998.
U. Schaefer and H. Loth. An ex-vivo model for the study of drug penetration into human skin. Pharm. Res. 13(Suppl.):366 (1996).
R. L. Bronaugh, R. F. Stewart, and M. Simon. Methods for invitro percutaneous absorption studies VII: use of excised human skin. J. Pharm. Sci. 75:1094–1097 (1986).
S. M. Harrison, B. W. Barry, and P. H. Dugard. Effects of freezing on human skin permeability. J. Pharm. Pharmacol. 36:261–262 (1984).
R. G. van der Molen, F. Spies, J. M. van 't Noordende, E. Boelsma, A. M. Mommaas, and H. K. Koerten. Tape stripping of human stratum corneum yields cell layers that originate from various depths because of furrows in the skin. Arch. Dermatol. Res. 289:514–518 (1997).
V. P. Shah, G. L. Flynn, A. Yacobi, H. I. Maibach, C. Bon, N. M. Fleischer, T. J. Franz, S. A. Kaplan, J. Kawamoto, L. J. Lesko, J.-P. Marty, L. K. Pershing, H. Schaefer, J. A. Sequeira, S. P. Shrivastava, J. Wilkin, and R. L. Williams. Bioequivalance of topical dermatological dosage forms—methods of evaluation of bioequivalance. Pharm. Res. 15:167–171 (1998).
L. K. Pershing, J. Corlett, and C. Jorgensen. In vivo pharmacokinetics and pharmacodynamics of topical ketoconazole and miconazole in human stratum comeum. Antimicrob. Agents Chemother. 38:90–95 (1994).
W. A. Ritschel, A. Sabouni, and A. S. Hussain. Percutaneous absorption of coumarin, griseofulvin and propanolol across human scalp and abdominal skin. Meth. And Find. Exp. Clin. Pharmacol. 11:643–646 (1989).
R. Panchagnula, K. Stemmer, andW. A. Ritschel. Animal models for transdermal drug delivery. Methods Find. Exp. Clin. Pharmacol. 19:335–341 (1997).
T. Kimura, N. Nagahara, K. Hirabayashi, Y. Kurosaki, and T. Nakayama. Enhanced percutaneous penetration of flufenamic acid using lipid disperse systems containing glycosylceramides. Chem. Pharm. Bull. 37:454–457 (1989).
Y. Kurosaki, N. Nagahara, T. Tanizawa, H. Nishimura, T. Nakayama, and T. Kimura. Use of lipid disperse systems in transdermal drug delivery: Comparative study of flufenamic acid permeation among rat abdominal skin, silicon rubber membrane and stratum corneum sheet isolated from hamster cheek pouch. Int. J. Pharmaceut. 67:1–9 (1991).
A. Alonso, N. C. Meirelles, V. E. Yushmanov, and M. Tabak. Water increases the fluidity of intercellular membranes of stratum corneum: correlation with water permeability, elastic and electrical resistance properties. J. Invest. Dermatol. 106:1064–1069 (1996).
T. S. Wiedmann. Influence of hydration on epidermal tissue. J. Pharm. Sci. 77:1037–1041 (1988).
C. S. King, S. P. Barton, S. Nicholls, and R. Marks. The change in properties of the stratum corneum as a function of depth. Br. J. Dermatol. 100:165–172 (1979).
S. J. Chapman, A. Walsh, S. M. Jackson, and P. S. Friedmann. Lipids, proteins and corneocyte adhesion. Arch. Dermatol. Res. 283:167–173 (1991).
G. Hauck. Hornschichtlipide: Methoden zu ihrer Bestimmung sowie ihr Einfluß auf die Penetration von Flufenaminsäure in das Stratum Corneum. Dissertation Saarbruecken 1994.
F. Bonte, A. Saunois, P. Pinguet, and A. Meybeck. Existence of a lipid gradient in the upper stratum corneum and its possible biological significance. Arch. Dermatol. Res. 289:78–82 (1997).
Y. N. Kalia, F. Pirot, and R. H. Guy. Homogeneous transport in a heterogeneous membrane: water diffusion across human stratum corneum in vivo. Biophys. J. 71:2692–2700 (1996).
A. Rougier, D. Dupuis, C. Lotte, R. Roguet, R. C. Wester, and H. I. Maibach. Regional variation in percutaneous absorption in man: Measurement by the stripping method. Arch. Dermatol. Res. 278:465–469 (1986).
M. A. Lampe, A. L. Burlingame, J. A. Whitney, M. L. Williams, B. E. Brown, E. Roidman, and P. M. Elias. Human stratum corneum lipids: characterization and regional variation. J. Lipid. Res. 24:120–130 (1983).
P. M. Elias, E. R. Cooper, A. Korc, and B. E. Brown. Percutaneous transport in relation to stratum corneum structure and lipid composition. J. Invest. Dermatol. 76:297–301 (1981).
Y. Seta, A. H. Ghanem, W. I. Higuchi, S. Borsadia, C. R. Behl, and A. W. Malick. Physical model approach to understanding finite dose transport and uptake of hydrocortisone in hairless guinea-pig skin. Int. J. Pharm. 81:89–99 (1992).
Rights and permissions
About this article
Cite this article
Wagner, H., Kostka, KH., Lehr, CM. et al. Drug Distribution in Human Skin Using Two Different In Vitro Test Systems: Comparison with In Vivo Data. Pharm Res 17, 1475–1481 (2000). https://doi.org/10.1023/A:1007648807195
Issue Date:
DOI: https://doi.org/10.1023/A:1007648807195