Abstract
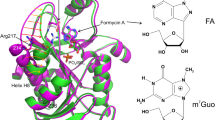
Three-dimensional structures are known from X-ray studies of the nucleoside diphosphate(NDP) kinase of many organisms from bacteria to human. All NDP kinases have subunits ofabout 150 residues with a very similar fold based on the αβ sandwich orferredoxin fold.This fold is found in many nucleotide or polynucleotide-binding proteins with no sequencerelationship to NDP kinase. This common fold is augmented here with specificfeatures: asurface α-helix hairpin, the Kpn loop, and the C-terminal extension. The α-helix hairpin andKpn loop make up the nucleotide binding site, which is unique to NDP kinase and differentfrom that of other kinases or ATPases. The Kpn loop and the C-terminal extension are alsoinvolved in the quaternary structure. Whereas all known eukaryotic NDP kinases, includingmitochondral enzymes, are hexamers, some bacterial enzymes are tetramers. However,hexameric and tetrameric NDP kinases are built from the same dimer. The structural environmentof the active histidine is identical in all. The nucleotide binding site is also fully conserved,except for a feature implicating C-terminal residues in the hexamer, but not in the tetramer.Structural data on the native and phosphorylated enzyme, complexes with substrates, inhibitor,and a transition state analog, give a solid basis to a mechanism of phosphate transfer in whichthe largest contributors to catalysis are the 3′-OH of the sugar and the bound Mg2+ in thenucleotide substrate. In contrast, we still lack structural data relating to DNA binding andother functions of NDP kinases.
Similar content being viewed by others
REFERENCES
Adman, E. T., Sieker, L. C., and Jensen, L. H. (1973). J. Biol. Chem. 248, 3987-3996.
Admiraal, S. J., Schneider, B., Meyer, P., Janin, J., Véron, M., Deville-Bonne, and Herschlag, D. (1999). Biochemistry 38, 4701-4711.
Biggs, J., Hersperger, E., Steeg, P. S., Liotta, L. A., and Shearn, A. (1990). Cell 63, 933-940.
Biondi, R. M., Schneider, B., Passeron, E., and Passeron, S. (1998). Arch. Biochem. Biophys. 353, 85-92.
Bourdais, J., Biondi, R, Sarfati, S., Guerreiro, C., Lascu, I., Janin, J., and Véron, M. (1996). J. Biol. Chem. 271, 7887-7890.
Bryant, S. H., Madej, T., Janin, J., Liu, Y., Ruoho, A. E., Zhang, G., and Hurley, H. (1997). Nature (London) 388, 34.
Cherfils, J., Moréra, S., Lascu, I, Véron, M., and Janin, J. (1994). Biochemistry 33, 9062-9069.
Chiadmi, M., Moréra, S., Lascu, I., Dumas, C., LeBras, G., Véron, M., and Janin, J. (1993). Structure 4, 283-293.
De la Rosa, A., Williams, R. L., and Steeg, P. S. (1995). Bioessays 17, 53-62.
Dumas, C., Lascu, I., Moréra, S., Glaser, P., Fourme, R., Wallet, V., Lacombe, M. L., Véron, M., and Janin, J. (1992) EMBO J. 11, 3203-3208.
Giartoso, A., Erent, M., Cervoni, L., Moréra, S., Janin, J., Konrad, M., and Lascu, I. (1996). J. Biol. Chem. 271, 17845-17851.
Gilles, A. M., Presecan, E., Vonica, A., and Lascu, L. (1991). J. Biol. Chem. 266, 8784-8789.
Gonin, P., Xu, Y., Milon, L., Dabernat, S., Morr, M., Kumar, R., Lacombe, M. L., and Lascu, L. (1999). Biochemistry 38, 7265-7272.
Herzberg, O., Reddy, P., Sutrina, S., Saier, M. H. J., Reizer, J., and Kapadia, P. (1992). Proc. Nat. Acad. Sci. USA 89, 2499-2503.
Janin, J., Miller, S., and Chothia, C. (1988). J. Mol. Biol. 204, 155-164.
Janin, J. (1993). Nature 365, 21.
Karlsson, A., Mesnildrey, S., Xu, Y., Moréra, S., Janin, J., and Véron, M. (1996). J. Biol. Chem. 271,19928-19934.
Kinoshita, K., Sadanami, K., Kidera, A., and Go, N. (1999).Protein Eng. 12, 11-14.
Ladher, J. E., Abdulaev, N. G., Kakuev, D. L., Tordova, M., Ridge, K. D., and Gilliland, G. L. (1999). Acta Crystallog. D55, 1127-1135.
Lascu, L., Chafotte, A., Limbourg-Bouchon, B., and Véron, M. (1992). J. Biol. Chem. 267, 12775-12781.
Lecroisey, A., Lascu, L, Bominaar, A., Véron, M., and Delepierre, M. (1995).Biochemistry 34, 12445-12450.
Meyer, P., Schneider, B., Sarfati, S., Deville-Bonne, D., Guerreiro, C., Boretto, J., Janin, J., Véron, M., and Canard, B. (2000). Embod. 19, 3520-3529.
Milon, L., Rousseau-Merck, M. F., Munier, A., Erent, M., Lascu., I., Capeau, J., and Lacombe, M. L. (1997). Human Genet. 99, 550-557.
Milon, L., Meyer, P., Chiadmi, M., Munier, A., Johansson, M., Karlsson, A., Lascu., I., Janin, J., Capeau, J., and Lacombe, M. L. (2000).J. Biol. Chem., in press.
Moodie, S. L., and Thornton, J. M. (1993). Nucl. Acid Res. 6, 1369-1380.
Min, K., Kim, S. Y., Song, H. K., Chang, C., Cho, S. J., Moon, J., Yang, J. K., Lee, J. Y., Lee, K. J., and Suh, S. W. (2000). Acta Crystallog. D56, 504-505.
Moréra, S., LeBras, G., Lascu, I., Lacombe, M. L., Vé ron, M, and Janin, J. (1994a). J. Mol. Biol. 243, 873-890.
Moréra, S., Lascu, I., Dumas, C., LeBras, G., Véron, M., and Janin, J. (1994b). Biochemistry 33, 459-467.
Moréra, S., Chiadmi, M., LeBras, G., Lascu, I., and Janin, J. (1995a). Biochemistry 34, 11062-11070.
Moréra, S., Lacombe, M. L., Xu, Y., LeBras, G., and Janin, J. (1995b). Structure 3, 1307-1314.
Okabe-Kado, J., Kasukabe, T., Hozumi, M., Honma, Y., Kimura, N., Baba, H., Urano, T., and Shiku, H. (1995). FEBS Lett. 363, 311-315.
Pastore, A., Saudek, V., Ramponi, G., and Williams, R. J. P. (1992). J. Mol. Biol. 224, 427-440.
Postel, E. H. (1998). Intern. J. Biochem. Cell Biol. 30, 1291-1295.
Schneider, B., Xu, Y., Sellam, O., Janin, J., Véron, M., and Deville-Bonne, D. (1998a). J. Biol. Chem. 273, 28773-28778.
Schneider, B., Xu, Y., Sellam, O., Sarfati, R., Janin, J., Véron, M., and Deville-Bonne, D. (1998b). J. Biol. Chem. 273, 11491-11497.
Schulz, G. E. (1992). Current Opinion Struct. Biol. 2, 61-67.
Stahl, J. A., Leone, A., Rosengard, A. M., Porter, L., King, C. R. and Steeg, P. S. (1991). Cancer Res. 51, 445-449.
Strelkov, S. V., Perisic, O., Webb, P. A., and Williams, R. L. (1995). J. Mol. Biol. 249, 665-674.
Swindells, M. B., Orengo, C. A., Jones, D. T., Pearl, L. H., and Thornton, J. M. (1993). Nature (London) 365, 21.
Swindells, M. B., and Alexandrov, N. N. (1994). Nature Struct. Biol. 1, 677-678.
Tepper, A. D., Dammann, H., Bominaar, A. A., and Véron, M. (1994). J. Biol. Chem. 269, 1887-1890.
Webb, P. A., Perisic, O., Mendola, C. E., Backer, J. M., and Williams, R. L. (1995). J. Mol. Biol. 251, 574-587.
Williams, R. L., Oren, D. A., Munoz-Dorado, J., Inouye, S., Inouye, M., and Arnold, E. (1993). J. Mol. Biol. 234, 1230-1247.
Xiao, B., Shi, G., Chen, X., Yan, H., and Ji, X. (1999). Structure 7, 489-496.
Xu, Y., Moréra, S., Janin, J., and Cherfils, J. (1997a). Proc. Natl. Acad. Sci USA 94, 3579-3583.
Xu, Y., Sellam, O., Moréra, S., Sarfati, R., Véron, M., and Janin, J. (1997b). Proc. Natl. Acad. Sci USA 94, 7162-7165.
Xu, Y., Lecroisey, Y., Véron, M., Delepierre, M., and Janin, J. (1997c). Proteins 28, 150-152.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Janin, J., Dumas, C., Moréra, S. et al. Three-Dimensional Structure of Nucleoside Diphosphate Kinase. J Bioenerg Biomembr 32, 215–225 (2000). https://doi.org/10.1023/A:1005528811303
Issue Date:
DOI: https://doi.org/10.1023/A:1005528811303