Abstract
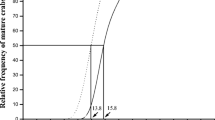
Population dynamics of Stylaria lacustris were analyzed over 2 years in a pond located at Los Talas, Argentina. In both years, the peak in abundance, due to intense asexual reproduction, fell at the end of winter, and was followed by mature individuals. The species was not collected during summer. Temperature was the main factor regulating the population through both sexual and asexual reproduction. In the second year, density was lower and correlated with a lower water level and a reduced vegetation development. Individuals were randomly distributed in periods of high abundance and contagious when density was low. Generation time was 15 days.
Similar content being viewed by others
References
Beckett, D. C., T. P. Aartila & A. C. Miller, 1992. Seasonal change in plant-dwelling Chironomidae and Naididae in a Wisconsin lake. J. Freshwat. Ecol. 7: 45–57.
Brinkhurst, R. O. & B. G. M. Jamieson, 1971. Aquatic Oligochaeta of the World. Oliver & Boyd, Edinburgh: 860 pp.
Brinkhurst, R. O. & M. R. Marchese, 1992. Guía para la identificación de oligoquetos acuáticos continentales de Sud y Centroamérica. Colección Climax No 6. Segunda edición: 207 pp.
Christensen, B., 1984. A sexual propagation and reproductive strategies in aquatic Oligochaeta. Hydrobiologia 115: 91–95.
Di Persia, D. H., 1980. The Aquatic Oligochaeta of Argentina: Current status of knowledge. In Brinkhurst, R. O. & D. G. Cook (eds), Aquatic Oligochaeta Biology. Plenum Press, New York: 79–113.
Elliott, J. M., 1983. Some methods for the statistical analysis of samples of benthic invertebrates. Freshwat. Biol. Assoc. Sci. Pub. 25, Ambleside: 156 pp.
Gavrilov, K., 1977. Oligochaeta. In Hurlbert, S. H. (ed.), Biota Acuática de Sudamérica Austral. San Diego State Univ., San Diego, California: 99–121.
Gluzman de Pascar, C., 1987. Aquatic Oligochaeta in some tributaries of the Río de La Plata, Buenos Aires, Argentina. Hydrobiologia 144: 125–130.
Gluzman, C., 1990. Freshwater Oligochaeta in stagnant water bodies in Buenos Aires Province, Argentina. Physis B 48: 47–50.
Gruffydd, Ll. D., 1965. The population biology of Chaetogaster limnaei limnaei and Chaetogaster limnaei vaghini (Oligochaeta). J. anim. Ecol. 34: 667–690.
Harman, W. J., R. O. Brinkhurst & M. Marchese, 1988. A contribution to the taxonomy of the Aquatic Oligochaeta (Naididae) of South America. Can. J. Zool. 66: 2233–2242.
Irmler, U., 1989. Population ecology and migration of Dero multibranchiata Stieren, 1892 (Naididae, Oligochaeta) in the Central Amazon inundation forest. Amazoniana 11: 31–52.
Jonásson, P. M., 1972. Ecology and production of the profundal benthos. Oikos Suppl. 14: 1–148.
Juget, J. & M. Lafont, 1994. Theoretical habitat templets, species traits, and species richness: aquatic oligochaetes in the Upper Rhône River and its floodplain. Freshwat. Biol. 31: 327–340.
Learner, M. A., G. Lochhead & B. D. Hughes, 1978. A review of the biology of British Naididae (Oligochaeta) with emphasis on the lotic environment. Freshwat. Biol. 8: 357–375.
Lochhead, G & M. A. Learner, 1983. The effect of temperature on asexual population growth of three species of Naididae (Oligochaeta). Hydrobiologia 98: 107–112.
Loden, M., 1981. Reproductive ecology of Naididae (Oligochaeta). Hydrobiologia 83: 115–123.
Marchese, M. R., 1986. Nuevos aportes al conocimiento de los Oligoquetos del Río Paraná Medio y algunos tributarios. Stud. Neotrop. Fauna Envir. 21: 231–249.
Pujals, M. A., 1989. Asociaciones de oligoquetos del pleuston en un canal artificial conexo al Río de la Plata en el Partido de Ensenada, Buenos Aires, Argentina. An. Soc. Cient. Arg. 219: 37–47.
Sampóns, M. R., 1989. Oligoquetos bentónicos del arroyo Rodríguez (Provincia de Buenos Aires). Neotropica 35: 101–112.
Schierwater, B. & C. Hauenschild, 1990a. A photoperiod determined life-cycle in an oligochaete worm. Biol. Bull. 178: 111–117.
Schierwater, B. & C. Hauenschild, 1990b. The position and consequences of a vegetative mode of reproduction in the life cycle of a hydromedusa and an oligochaete worm. In Hoshi, M. & O. Yamashita (eds), Advances in Invertebrate Reproduction 5. Elsevier Science Publishers B. V. (Biomedical Division): 37–42.
Sloreid, S. E., 1994. Oligochaete response to changes in water flow in the Dokka Delta, Lake Randsfjorden (Norway) caused by hydroelectric power development. Hydrobiologia 278: 243–249.
Smith, M. E., 1986. Ecology of Naididae (Oligochaeta) from an alkaline bog stream: life history patterns and community structure. Hydrobiologia 133: 79–90.
Timm, T., 1984. Potential age of Aquatic Oligochaeta. Hydrobiologia 115: 101–104.
Varela, M. E., 1990. Notas taxonómicas y ecológicas sobre algunos oligoquetos dulceacuícolas del nordeste argentino. 1. Naididae. Stud. Neotrop. Fauna Envir. 25: 223–233.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Armendáriz, L.C. Population dynamics of Stylaria lacustris (Linnaeus, 1767) (Oligochaeta, Naididae) in Los Talas, Argentina. Hydrobiologia 438, 217–226 (2000). https://doi.org/10.1023/A:1004139622036
Issue Date:
DOI: https://doi.org/10.1023/A:1004139622036