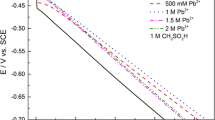
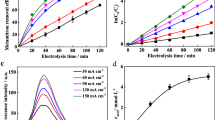
Abstract
The rate of hexavalent chromium regeneration from trivalent chromium, present as an impurity in a chromium plating solution at the Bi-doped PbO2 anode, is found to be approximately four times greater than the corresponding rate observed for a PbO2 coated lead (anodized lead) anode. A mathematical model that takes into account species electromigration and associated mass transfer effects was developed and tested. Dynamic concentration data for various chromium species were used along with the mathematical model to evaluate the various rate parameters.
Similar content being viewed by others
References
N.V. Mandich, Plat. Surf. Finish. 84 (6) (1997) 67.
N.V. Mandich, ‘Removal of Metallic Impurities from Plating Solutions by Electrocoagulation', AESF Chromium Colloquium, Vol. 36 (AESF, Orlando, FL, Jan. 1994).
S.B. Lalvani and N.V. Mandich, ‘Removal of Metallic Impurities in Chromium Plating Solutions by Electrocoagulation', Illinois Waste Management and Research Center, Champaign, IL, Project 97025 (1997).
N.V. Mandich, C-C. Lee and J.R. Selman, Plat. Surf. Finish. 84 (12) (1997) 82.
S.L. Guddati, T.M. Holsen, C-C. Li, J.R. Selman and N.V. Mandich, J. Appl. Electrochem. 29 (1999) 1129.
S.B. Lalvani and N.V. Mandich, ‘Removal of Metallic Impurities in Chromium Plating Solutions by Electrocoagulation-Phase I', Project No. 97025, Final Report to Illinois Waste Management and Research Center, Champaign, IL (1998).
J. Pattanayak, K. Mondal, N.V. Mandich, T. Wiltowski and S.B. Lalvani, Metal Finish. 98 (3) (2000) 39.
J. Pattanayak, N.V. Mandich, K. Mondal, T. Wiltowski and S.B. Lalvani, Environmental Technol. 20 (1999) 317.
S.B. Lalvani, K. Mondal, J. Pattanayak, N.V. Mandich and T. Wiltowski, J. Electrochem. Soc., under review.
K.L. Pamplin and D.C. Johnson, J. Electrochem. Soc. 143 (1996) 2119.
A.J. Bard and L.R. Faulkner, ‘Electrochemical Methods-Fundamentals and Applications’ (J. Wiley & Sons, New York, 1980).
R.J. Vora, S.R. Taylor and G.E. Stoner, in F. Hine et al., ‘Factors A€ecting the Performance of PbO2 Anodes in the Generation of Hexavalent Chromium’ Proceedings of the Symposium on Performance of Electrodes for Industrial and Chemical Processes (The Electrochemical Society, 1989), p. 279.
D.A. Ratkowsky, ‘Nonlinear Regression Modeling: A Uniffied Practical Approach’ (Marcel Dekker, New York, 1983).
S.L. Guddati, T.M. Holsen and J.R. Selman, ‘Optimization of Porous Ceramic Diaphragm Cell Operation for the Removal of Metallic Impurities from Chrome Plating Bath', Report for Summer Research Grant Program (AESF, Orlando, FL, 1995).
R.E. Treybal, ‘Mass Transfer Operations', 3rd edn (McGraw-Hill, London, 1980), pp 35–37.
Y-L. Hsiao and D.C. Johnson, J. Electrochem. Soc. 136 (1989) 3704.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mondal, K., Mandich, N. & Lalvani, S. Regeneration of hexavalent chromium using a Bi-doped PbO2 anode. Journal of Applied Electrochemistry 31, 165–173 (2001). https://doi.org/10.1023/A:1004107117531
Issue Date:
DOI: https://doi.org/10.1023/A:1004107117531