Abstract
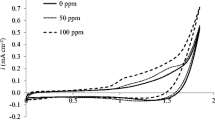
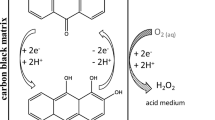
Hydrogen peroxide production by the intermediate electroreduction of the 2-ethyl–9,10-anthraquinone (EAQ) was carried out in a flow-by cell and a two-phase electrolyte formed by a mixture of tributylphosphate (TBP) and diethylbenzene (DEB) as the organic phase, and a solution of NaOH as the aqueous phase. The cathode used was a reticulated vitreous carbon (RVC) foam. We have examined the following process variables: electrolysis current (0.3–3.1A), catholyte flow rate (470–1630mlmin−1), EAQ concentration in the organic phase (0.21–0.42m), organic/aqueous phase volume ratio (1/9–4/6) and grade of porosity of the RVC (20–45ppi). The electrolyses can be carried out in the presence or absence of oxygen gas. The first method is the so-called ‘one-step electrolysis’ and the second method is the ‘two-step electrolysis’. In the second method, the disodium salt of the hydroquinone (EAQNa2) is electrochemically formed in the absence of oxygen. The second step consists of the chemical reaction of this salt with oxygen to form hydrogen peroxide. We obtained a hydrogen peroxide concentration of 0.8m after 10Ah with an electrolysis current of 1.55A and a current efficiency of 70%.
Similar content being viewed by others
References
J.-P. Schirmann and S. Y. Delavarenne, ‘Hydrogen peroxide in organic chemistry’ Special print for Elfa Ox-ychemie Handels AG, S.E.T.E. Publ., (1979).
Ullmann's, ‘Encyclopedia of Industrial Chemistry’, Vol. A13, 5th edn., VCH, New York (1989), p. 443.
Kirk Othmer, ‘Encyclopedia of Chemical Technology’, Vol. 13, 3rd edn., J. Wiley & Sons, New York. (1981), p. 12.
R. J. Taylor and A. A. Humffray, J. Electroanal. Chem. 64 (1975) 85.
E. Yeager, P. Krouse and K. V. Rao, Electrochim. Acta 9 (1964) 1057.
I. Morcos and E. Yeager, ibid. 15 (1970) 953.
R. J. Taylor and A. A. Humffray, J. Electroanal. Chem. 64 (1975) 63.
A. A. Humffray) idem, ibid. 64 (1975) 95.
C. Oloman, J. Electrochem. Soc. 126 (1979) 1885.
C. Oloman and A. P. Watkinson, J. Appl. Electrochem. 9 (1979) 117.
A. Clifford, D. Dong, E. Giziewicz and D. Rogers, Ex-tended Abstract of the Spring Meeting of the Electro-chemical Society, May 8 (1990).
J. A. McIntyre, Interface 4(1), (1995) 29.
[13] S. Lynn and H. H. Paalman, British Patent 1 154 096 (1967).
[14] S. Lynn and H. H. Paalman, US Patent. 3 351 104 (1970).
G. S. Calabrese and M. S. Wrighton, J. Electrochem. Soc. 128 (1981) 1014.
B. Keita and L. Nadjo, J. Electroanal. Chem. 145 (1983) 431.
R. F. Knarr, M. Velasco, S. Lynn and C. W. Tobias, J. Electrochem. Soc. 139 (1992) 948.
A. Huissoud and P. Tissot, J. Appl. Electrochem., 29 (1999) 11.
W. F. Schumb, C. N. Satterfield and R. L. Wentworth, ‘Hydrogen peroxide’, Reinhold, New York (1955), p. 561.
[20] Unpublished results.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Huissoud, A., Tissot, P. Electrochemical reduction of 2-ethyl-9,10-anthraquinone (EAQ) and mediated formation of hydrogen peroxide in a two-phase medium Part II: Production of alkaline hydrogen peroxide by the intermediate electroreduction of EAQ in a flow-by porous electrode in two-phase liquid–liquid flow. Journal of Applied Electrochemistry 29, 17–25 (1999). https://doi.org/10.1023/A:1003417632288
Issue Date:
DOI: https://doi.org/10.1023/A:1003417632288