Abstract
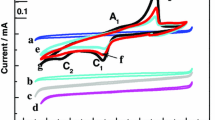
The electrochemical behaviour of UIII in molten salts was studied at 715 ∘C under argon atmosphere in a molten equimolar NaCl–KCl mixture containing uranium ions in oxidation state III (UCl3). Three different complementary electrochemical methods were used, namely cyclic voltammetry, chronopotentiometry and square wave voltammetry. These techniques provide data to explain the electroreduction mechanism. The reduction of UIII in the NaCl–KCl mixture occurs in a single step with an exchange of three electrons, this process is controlled by the diffusion of UIII. The diffusion coefficient of U(III) was calculated using the three electrochemical methods.
Similar content being viewed by others
References
M.V. Smirnov and O.V. Skiba, Electrochem. Molten and Solid Electrolytes 2 (1964) 12.
G. Boisde, G. Chauvin, H. Coriou and J. Hure, Electrochim. Acta 5 (1961) 54.
S. Flengas, Can. J. Chem. 39 (1961) 773.
B. Partridge, J. Inorg. Nucl. Chem. 19 (1961) 379.
M.V. Smirnov and O.V. Skiba, Trans. Inst. Electrochem. 2 (1964) 1.
O.V. Skiba, M.V. Smirnov and T.F. Khazemova, Electrochem. Molten and Solid Electrolytes 2 (1964) 7.
D.L. Hill, J. Perano and R.A. Osteryoung, J. Electrochem. Soc. 107 (1960) 698.
F. Caligara, L. Martinot and G. Duyckaerts, Bull. Soc. Chim. Belg. 75 (1967) 15.
D.S. Poa, Z. Tomczuk and R.K. Steunenberg, J. Electrochem. Soc. 135 (1988) 1161.
P. Chamelot, B. Lafage and P. Taxil, J. Electrochem. Soc. 143(5) (1996) 1570.
R.J. Gale and D.G. Lovering, 'Molten Salt Techniques' (Plenum Press, New York, 1984), p. 152.
A.T. Hubbard and F.C. Anson, Anal. Chem. 38 (1966).
C.R. Christensen and F.C. Anson, Anal. Chem. 35 (1963).
L. Martinot, J. Inorg. Nucl. Chem. 37 (1978) 2525.
A.J. Bard and R.L. Faulkner, 'Electrochimie: Principes, Methodes et Applications' (Masson, Paris, 1983).
H.J.S. Sand, Phil. Mag. 1 (1901) 45.
J.G. Osteryoung and J.J. O'Dea, Electroanal. Chem. 14 (1986) 209.
B.N. Kabanov, 'Electrochemistry of Metals and Adsorption' (Freund Publishing House, Israel, 1969), p. 3.
E. Laviron, Electroanal. Chem. Interfac. Electrochem. (Elsevier Sequoia S.A., Lausanne), 52 (1974) 355.
P. Chamelot, B. Lafage and P. Taxil, Electrochim. Acta 43 (5-6) (1997) 607.
G.C. Barker, Anal. Chim. Acta 18 (1958) 118.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Serrano, K., Taxil, P. Electrochemical reduction of trivalent uranium ions in molten chlorides. Journal of Applied Electrochemistry 29, 497–503 (1999). https://doi.org/10.1023/A:1003402029895
Issue Date:
DOI: https://doi.org/10.1023/A:1003402029895