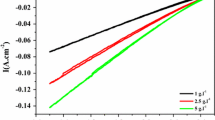
Abstract
Electrochemical methods, including polarization experiments and impedance spectroscopy, were used to evaluate the effectiveness of benzotriazole (BTA) in an aqueous solution of polyethylene glycol (PEG) in protecting polished archaeological copper or archaeological copper covered with corrosion products. The adsorption of PEG on the polished copper significantly limited the corrosion current. The presence of benzotriazole enhanced the protection of the polished copper, giving maximum protection at a concentration of 10−2 mol l−1 of BTA in 20 vol% PEG 400 solution. On the other hand, PEG solutions caused degradation of the corrosion products of the copper. This degradation increased with time. When BTA was added, the corrosion products were preserved and, the higher the BTA concentration, the more the corrosion current decreased. In PEG 400 solution protection of the corrosion products of the copper by BTA improved over time.
Similar content being viewed by others
References
D. Grattan, Studies in Conservation 27 (1982) 124–136.
I.D. MacLeod, Studies in Conservation 36 (1991) 222–234.
L.S. Selwyn, D.A. Rennie-Bisaillon and N.E. Binnie, Studies in Conservation 38 (1993) 180–197.
V. Agyropoulos, J.J. Rameau, F. Dalard and C. Degrigny, Submitted to Studies in Conservation.
C. Clerc and R. Alkire, J. Electrochem. Soc. 138 (1991) 25–33.
P.G. Fox, G. Lewis and P.J. Boden, Corros. Sci. 19 (1979) 457–467.
E.A.A. Ashour, S.M. Sayed and B.G. Ateya, J. Appl. Electrochem. 25 (1995) 137–141.
J.D. Reid and A.P. David, Plat. Surf. Finish. 74 (1987) 66–70.
J.P. Diard, B. Le Gorrec and C. Montella, ‘Cinétique Électrochimique’, (Des Sciences et Des Arts, Hermann, Paris, 1996), pp.85–97.
S. Kerit, F. Chaouket, A. Srihiri and M. Keddam, J. Appl. Electrochem. 24 (1994) 1139–45.
S.E. Faidi and J.D. Scantlebury, J. Electrochem. Soc. 136 (1989) 990–95.
A.M. Beccaria and C. Bertolotto, Electrochim. Acta 42 (1997) 1361–71.
E. Ahlberg and M. Friel, Electrochim. Acta 34 (1989) 1523–28.
Y.Z. Wang, A.M. Beccaria and G. Poggi, Corros. Sci. 36 (1994) 1277–88.
M. Pourbaix, ‘Atlas d'Équilibres Électrochimiques’ (Gauthier-Villars, Paris, 1963), p. 387.
A. Bouridah, ‘Contribution à l'Étude des PropriÉtés de Transport Ionique d'Électrodes Polymè res: les Polyèthers-sels de Lithium’ Thèse de l'Institut National Polytechnique de Grenoble, France, June 1986.
L.B. Brostoff, ‘Metal 95’, James & James, 1997 pp. 99–108.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Guilminot, E., Rameau, JJ., Dalard, F. et al. Benzotriazole as inhibitor for copper with and without corrosion products in aqueous polyethylene glycol. Journal of Applied Electrochemistry 30, 21–28 (2000). https://doi.org/10.1023/A:1003400516421
Issue Date:
DOI: https://doi.org/10.1023/A:1003400516421