Abstract
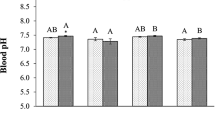
A 15 month long experiment was undertaken to document responses of the Salton Sea biota to experimentally manipulated salinity levels (30, 39, 48, 57, and 65 g l-1) in 312-liter fiberglass tanks maintained outdoors. At two salinities (39 and 57 g l-1) microcosms were set up each having one small tilapia ( Oreochromis mossambicus) in order to assess its influence on the system. To 28 tanks filled with Salton Sea water diluted to 30 g l-1, different salts (NaCl, Na2SO_4, MgSO4 · 7H2O, KCl) were added in constant proportions to produce the desired salinity levels. Salton Sea shoreline sediment was added to the bottom of each tank, and inocula of algae and invertebrates were added on several occasions. Invertebrate populations, phytoplankton, periphyton, and water chemistry were monitored at regular intervals. This article present the results concerning water chemistry and nutrient cycling. There was no apparent increase in salinity over time, though ∼ 1190 l of tapwater with a salinity of ∼ 0.65 g l-1 were added to each tank during the experiment. Ionic composition varied both among treatments and over time to some degree. Ca2 concentrations were the same at all salinities, while K1 concentrations were >3 times greater at the highest salinity than at the lowest. pH showed little consistent variation among salinities until the last few months when it was higher by ∼ 0.4 units at the two higher salinities than at the lower ones; it was unaffected by fish. Absolute oxygen concentrations were negatively correlated with salinity, and occasionally depressed by the presence of fish. PO3-4, dissolved organic phosphorus, and particulate phosphorus concentrations were often reduced by 30–80% at 65 g l-1 relative to lower salinities and by the presence of fish. Early in the experiment NO2-3 concentrations were >2 times higher at 57 and 65 g l-1 than at lower salinities, but otherwise effects of salinity on dissolved forms of nitrogen were not marked; particulate nitrogen was much lower at 65 g l-1 than at other salinities and also was reduced by up to 90% by the presence of fish. Silica concentrations increased over time at all salinities, but, relative to those at lower salinities, were reduced by 60–90% at 65 g l-1 by abundant periphytic diatoms. The TN:TP ratio (molar basis) was 24–30 initially and 35–110 at the end of the experiment; it was positively correlated with salinity and the presence of fish. Mechanisms accounting for the above patterns involve principally the biological activities of phytoplankton and periphyton, as modified by grazing by Artemia franciscana and Gammarus mucronatus, and the feeding and metabolic activities of the tilapia. The large reduction in water column TN and TP levels brought about by the fast-growing, phyto- and zooplanktivorous tilapia suggest that amelioration of the Salton Sea's hypereutrophic state might be assisted by a large scale, sustained yield fish harvesting operation.
Similar content being viewed by others
References
APHA, 1985. Standard methods for the examination of water and wastewater, 16th edn., American Public Health Association, Washington, DC.
Atkinson, M. J., 1987. Low Phosphorus sediments in a Hypersaline Marine bay. Estuarine coast. shelf Sci. 24: 335–347.
Bierhuizen, J. F. H. & E. E. Prepas, 1985. Relationship between nutrients, dominant ions, and phytoplankton standing crop in Prairie Saline Lakes. Can. J. Fish. aquat. Sci. 42: 1588–1594.
Black, G. F., 1983. Prognosis for water conservation and the development of energy resources at the Salton Sea: Destruction of preservation of this ecosystem? In V. D. Adamas & V. A. Lamarra (eds), Aquatic Resources Management of the Colorado River Ecosystem. Ann Arbor Science, Ann Arbor, Michigan: 363–382.
Black, G. F., 1988. Description of the Salton Sea sport fishery 1982– 1983. California Fish and Game Administrative Report 88–9.
Boyd, C. E. & B. W. Green, 1998. Dry matter, ash, and elemental composition of pond-cultured tilapia (Oreochromis aureus and O. niloticus). J. World Aquacult. Soc. (in press).
Brooks, J. L., 1969. Eutrophication and changes in the composition of the zooplankton. In Eutrophication: causes, consequences, correctives. National Academy of Sciences, Washington, D.C.: 236–255.
Carlander, K. D., 1955. The standing crop of fish in lakes. J. Fish. res. Bd Can. 12: 543–570.
Carpelan, L. H., 1961. Physical and chemical characteristics. In B. D. Walker (ed.), The Ecology of the Salton Sea. Calif. Fish Game, Fish Bull. 113: 17–32.
Carpenter, S. R. & J. F. Kitchell (eds), 1993. The Trophic Cascade in Lakes.Cambridge University Press, Cambridge, 385 pp.
Caraco, N., A. Tamse, O. Boutros & I. Valiela, 1987. Nutrient limitation of phytoplankton growth in brackish coastal ponds. Can. J. Fish. aquat. Sci. 44: 473–476.
Caraco, N., F. J. J. Cole & G. E. Likens, 1989. Evidence of sulphate controlled phosphorus release from sediments of aquatic systems. Nature 341: 316–318.
Clavero, V., A. Fernández & F. X. Niell, 1990. Influence of salinity on the concentration and rate of interchange of dissolved phosphate between water and sediment in Fuente Piedra lagoon (S. Spain). Hydrobiologia 197: 91–97.
Clavero, V., M. García, J. A. Fernández & F. X. Niell, 1993. Adsorption-desorption of phosphate and its availability in the sediment of a saline lake (Fuente de Piedra, southern Spain). Int. J. Salt Lake Res. 2: 153–163.
Conley, D. J., S. S. Kilham & E. Theriot, 1989. Differences in silica content between marine and freshwater diatoms. Limnol. Oceanogr. 34: 205–213.
Culkin, F. & R. A. Cox, 1966. Sodium, potassium, magnesium, calcium and strontium in the sea water. Deep Sea Res. 13: 789–804.
de Moor, F. C., R. C. Wilkinson & H. M. Herbst, 1986. Food and feeding habits of Oreochromis mossambicus (Peters) in hypertrophic Hartbeestpoort Dam, South Africa. S. Afr. J. Zool. 21: 170–176.
Downing, J. A. & E. McCauley, 1992. The nitrogen: phosphorus relationship in lakes. Limnol. Oceanogr. 37: 936–945.
Egge, J. K. & D. L. Aksnes, 1992. Silicate as regulating nutrient in phytoplankton competition. Mar. Ecol. Prog. Ser. 83: 281–289.
Galat, D. L. & R. Robinson, 1983. Predicted effects of increasing salinity on the crustacean zooplankton community of Pyramid Lake, Nevada. Hydrobiologia 105: 115–131.
Galat, D. L., M. Coleman & R. Robinson, 1988. Experimental effects of elevated salinity on three benthic invertebrates in Pyramid Lake, Nevada. Hydrobiologia 158: 133–144.
Gardner, W. S., S. P. Seitzinger & J. M. Malczyk, 1991. The effects of sea salts on the forms of nitrogen released from estuarine and freshwater sediments: Does ion pairing affect ammonium flux? Estuaries 14: 157–166.
Greenwald, G. M. & S. H. Hurlbert, 1993. Microcosm analysis of salinity effects on coastal lagoon plankton assemblages. Hydrobiologia 267: 307–335.
González, M. R., C. M. Hart, E. P. Simpson, R. Lara & S. H. Hurlbert, 1998. Salinity and fish effects on Salton Sea microecosystems: Phytoplankton and periphyton (in preparation).
Hammer, U. T., 1981. Primary production in saline lakes. A review. Hydrobiologia 81: 47–57.
Hammer, U. T., 1986. Saline lake ecosystems of the world. Dr W. Junk Publishers, Dordrecht, 616 pp.
Hart, C. M., 1994. Salinity and fish effects on invertebrates of the Salton Sea: A microcosm experiment. M.S. Thesis, SDSU, San Diego, 102 pp.
Hart, C., M. R. González, E. P. Simpson & S. H. Hurlbert, 1998. Salinity and fish effects on Salton Sea microecosystems: zooplankton and nekton. Hydrobiologia 381: 129–152.
Hecky, R. E. & P. Kilham, 1988. Nutrient limitation of phytoplankton in freshwater and marine environments: a review of recent evidence on the effects of enrichment. Limnol. Oceanogr. 33: 796–822.
Helsel, D. R. & R. M. Hirsch, 1992. Statistical Methods in Water Resources. Elsevier, New York, 522 pp.
Howarth, R. W., R. Marino, J. J. Cole & J. Lane, 1988. Nitrogen fixation in freshwater, estuarine and marine ecosystems. 1. Rates and importance. Limnol. Oceanogr. 33:688–701.
Hrbacek, J. M., V. Dvorkova, V. Korinek & L. Prochazkova, 1961. Demonstration of the effects of the fish stock on the species composition of the zooplankton and the intensity of the whole plankton association. Verh. int. Ver. Limnol. 14: 192–195.
Hurd, D. C., 1983. Physical and chemical properties of siliceous skeletons. In S. R. Aston (ed.), Silicon Geochemistry and Biogeochemistry. Academic Press, London: 187–244.
Hurlbert, S. H., J. Zedler & D. Fairbanks, 1972. Ecosystem alteration by mosquitofish (Gambusia affinis) predation. Science 175: 639–641.
Hutchinson, G. E., 1957. A Treatise on Limnology, vol. I, Geography, Physics, and Chemistry. Wiley, New York, 1115 pp.
Kitchell, J. F., J. F. Koonce & P. S. Tennis, 1975. Phosphorus flux through fishes. Verh. int. Ver. Limnol. 19: 2478–2484.
Kitchell, J. F., S. R. Carpenter, J. G. Hodgson, X. He & P. A. Soranno, 1993.Phosphorus in food webs: compensatory responses in experimental lakes. Verh. int. Ver. Limnol. 25: 344–34.
Lazzaro, X., 1987. A review of planktivorous fishes: their evolution, feeding behaviors, selectivities, and impacts. Hydrobiologia146: 97–167.
Mazumder, A., W. D. Taylor, D. J. Mcqueen & D. R. S. Lean, 1989. Effects of fertilization and planktivorous fish on epilimnetic phosphorus and phosphorus sedimentation in large eclosures. Can. J. Fish. aquat. Sci. 46: 1735–1742.
Maitipe, P. & S. S. De Silva, 1985. Switches between zoophagy, phytophagy and detritivory of Sarotherodon mossambicus (Peters) populations in twelve man-made Sri Lankan lakes. J. Fish. Biol. 26: 49–61.
Melack, J. M., P. Kilham & T. R. Fisher, 1982. Responses of phytoplankton to experimental fertilization with ammonium and phosphate in an African soda lake. Oecologia 52: 321–326.
McQueen, D. J., J. R. Post & E. L. Mills, 1986. Trophic relationships in freshwater pelagic ecosystems. Can. J. Fish. aquat. Sci. 43: 1571–1581.
Nakashima, B. S. & W. C. Leggett, 1980. The role of fishes in the regulation of phosphorus availability in lakes. Can. J. Fish. aquat. Sci. 37: 1540–1549.
Northcote, T. G.,1988. Fish in the structure and function of freshwater ecosystems: a ‘top-down’ view. Can. J. Fish. aquat. Sci. 45: 361–379.
O.E.E.S., 1995. Salton Sea management project: draft evaluation of salinity and elevation management alternatives. Ogden Environmental and Energy Services, San Diego, California.
Parsons (Parsons Water Resources, Inc.), 1986. Environmental Impact Report (EIR) Draft. Imperial Irrigation District Appendices A-I. California State Clearinghouse No. 86012903.
Por, F. D., 1980. A classification of hypersaline waters, based on trophic criteria. Mar. Ecol. 1: 121–131.
Post, J. R. & D. J. Mcqueen, 1987. The impact of planktivorous fish on the structure of a plankton community. Freshwat. Biol. 17: 79–89.
Schroeder, R. A., M. Rivera et al., 1993. Physical, chemical, and biological data for detailed study of irrigation drainage in the Salton Sea area, California, 1988–90. U.S. Geol. Survey Open-File Report 93–83.
Seitsinger, S. P., W. S. Gardner & A. K. Spratt, 1991. The effect of salinity on ammonium sorption in aquatic sediments: Implications for benthic nutrient recycling. Estuaries 14: 167–174.
Setmire, J. G., J. C. Wolfe & A. K. Stroud, 1990. Reconnaissance investigation of water quality, bottom sediment, and biota associated with irrigation drainage in the Salton Sea area, California, 1986–87. U.S. Geol. Surv. Water-Resources Investigations Report 89–4102, 68 pp.
Shapiro, J. & D. I. Wright, 1984. Lake restoration by biomanipulation: Round Lake, Minnesota, the first two years. Freshwat. Biol. 14: 371–383.
Sherwood, J. E.,F. Stagnitti, M. J. Kokkinn & W. D. Williams, 1992. A standard table for predicting equilibrium dissolved oxygen concentrations in salt lakes dominated by sodium chloride. Int. J. Salt Lake Res. 1: 1–6.
Simpson, E. P., 1994. Salinity and fish effects on the Salton Sea benthos. MS Thesis. San Diego State University, San Diego, California, 116 pp.
Simpson, P. & M. R. González, C. Hart & S. H. Hurlbert, 1998. Salinity and fish effects on Salton Sea microecosystems: benthos. Hydrobiologia 381: 153–177.
Simpson, P. & S. H. Hurlbert, 1998. Salinity effects on the growth, mortality and shell strength of Balanus amphitrite from the Salton Sea, California. Hydrobiologia 381: 179–190.
Sommer, U., 1988. Growth and survival strategies of planktonic diatoms. In C. D. Sandgren (ed.), Growth and Reproductive Strategies of Freshwater Phytoplankton. Cambridge, New York: 227–260.
Soto, D. & S. H. Hurlbert, 1991. Long-term experiments on calanoid-cyclopoid interactions. Ecol. Monogr. 61: 245–265.
Spencer, S. R., 1983. Marine biogeochemistry of silicon. In S. R. Ashton (ed.), Silicon Geochemistry and Geochemistry. Academic Press, New York: 101–142.
Stephens, D. W. & D. M. Guillespie, 1976. Phytoplankton production in the Great Salt Lake, Utah, and a laboratory study response to enrichment. Limnol. Oceanogr. 21: 74–87.
Threlkeld, S. T., 1988. Planktivory and planktivore biomass effects on zooplankton, phytoplankton, and the trophic cascade. Limnol. Oceanogr. 33: 1362–1375.
Tominaga, H., N. Tominaga & W. D. Williams, 1987. Concentration of some inorganic Plant Nutrients in saline lakes on the Yorke Peninsula, South Australia. Aust. J. mar. freshwat. Res. 38: 301–305.
U.S.D.I., 1970. Salton Sea California: water quality and ecological management considerations. U.S. Dept. Interior, Federal Water Quality Administration, Pacific Southwest Region, 53 pp.
Walker, B.W. (ed.), 1961. The Ecology of the Salton Sea, California in Relation to the Sport Fishery. Calif. Fish and Game Bull. 113: 1–204.
Weiderholm, 1980. Effects of dilution on the benthos of an alkaline lake. Hydrobiologia 68: 199–207.
Wurtsbaugh, W. A., 1992. Food-web modification by an invertebrate predator in the Great Salt Lake (USA). Oecologia 89: 168–175.
Wurtsbaugh, W. A. & T. S. Berry, 1990. Cascading effects of decreased salinity on the plankton, chemistry, and physics of the Great Salt Lake (Utah). Can. J. Fish. aquat. Sci. 47: 100–109.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
González, M.R., Hart, C.M., Verfaillie, J.R. et al. Salinity and fish effects on Salton Sea microecosystems: water chemistry and nutrient cycling. Hydrobiologia 381, 105–128 (1998). https://doi.org/10.1023/A:1003227624686
Issue Date:
DOI: https://doi.org/10.1023/A:1003227624686