Abstract
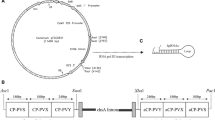
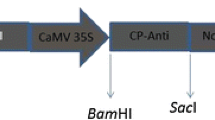
The Bzura commercial potato cultivar was transformed by sense or antisense constructs which included the coat protein gene of potato leafroll virus RNA. In the sense construct, the coat protein gene was preceded by a leader sequence shorter than that in the subgenomic RNA formed in infected cells. The antisense construct consisted of a sequence complementary to the first 2020 nucleotides of the subgenomic RNA. Selected transformants expressing viral RNA were resistant to virus challenge by viruliferous aphids. In one line, expression of the antisense RNA prevented virus infection even after grafting with scions from infected plants and therefore this transformant might be regarded as virus immune.
Similar content being viewed by others
References
Barker H, Reavy B, Kumar A, Webster KD and Mayo MA (1992) Restricted virus multiplication in potatoes transformed with the coat protein gene of potato leafroll luteovirus: similarities with a type of host gene-mediated resistance. Ann appl Biol 120: 55-64
Beachy RN, Loesch-Fries S and Tumer NE (1990) Coat protein mediated resistance against virus infection. Ann Rev Phytopathol 28: 451-474
Cuozzo M, O'Connell KM, Kaniewski W, Fang RX, Chua N-H and Rumer NE (1988) Viral protection in transgenic tobacco plants expressing the cucumber mosaic virus coat protein or its antisense RNA. Bio/Technology 6: 549-557
Derrick PM and Barker H (1992) The restricted distribution of potato leafroll luteovirus antigen in potato plants with transgenic resistance resembles that in clones with one type of host gene-mediated resistance. Ann Appl Biol 120: 451-457
Doyle JJ and Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue”. Phytochemical Bulletin 19: 11-15
Fang G and Grumet R (1993) Genetic engineering of potyvirus resistance using constructs derived from the zuccHini yellow mosaic virus coat protein gene. Mol Plant Microbe Interact 6: 358-367
Farinelli L and Malnoe P (1993) Coat protein gene-mediated resistance to potato virus Y in tobaco: Examination of the resistance mechanism-Is the transgenic coat protein required for protection? Mol Plant Microbe Interact 6: 284-292
Greene AE and Allison RF (1994) Recombination between viral RNA and transgenic plant transcripts. Science 263: 1423-1425
Hackland AF, Rybicki EP and Thomson JA (1994) Coat protein-mediated resistance in transgenic plants. Arch Virol 139: 1-22
Hammond J and Kamo KK (1995) Effective resistance to potyvirus infection conferred by expression of antisense RNA in transgenic plants. Mol Plant Microbe Interact 8: 674-682
Huntley C and Hall TC (1993a) Minus sense transcripts of brome mosaic virus RNA-3 intercistronic region interfere with viral replication. Virology 192: 290-297
Huntley C and Hall TC (1993b) Interference with bromemosaic virus replication by targeting theminus strand promoter. J Gen-Virol 74: 2445-2452
Kawchuk LM, Martin RR and Mc-Pherson J (1990) Resistance in transgenic potato expressing the potato leafroll virus coat protein gene. Mol Plant Microbe Interact 3: 301-307
Kawchuk LM, Martin RR and Mc-Pherson J (1991) Sense and anti-sense RNA-mediated resistance to potato leafroll virus in Russet Burbank potato plants. Mol Plant Microbe Interact 4: 247-253
Keese P., Martin RR, Kawchuk LM, Waterhouse PM and Gerlach WL (1990) Nucleotide sequences of an Australian and a Canadian isolate of potato leafroll luteovirus and their relationship with two European isolates. J GenVirol 71: 719-724
Maniatis T, Frisch EF and Sambrook J (1990) Molecular Cloning: a laboratory manual. Cold Spring Harbor Laboratory Cold Spring Harbor New York USA
Mayo MA, Robinson DJ, Jolly CA and Hyman L (1989) Nucleotide sequence of potato leafroll luteovirus RNA. J Gen Virol 70: 1037- 1051
McKinney H (1929) Mosaic diseases in the Canary Islands West Africa and Gibraltar. J Agric Res 39: 557-578
Miller JS and Mayo MA (1991) The location of the 5′ end of the potato leafroll luteovirus subgenomic coat protein RNA. J Gen Virol 72: 2633-2638
Nadolska-Orczyk AL., Miłkowska A, Pałucha A, Czembor P and OrczykW(1994) Regeneration and transformation of Polish cultivars of potato. Acta Societatis Botanicorum Poloniae Vol 6 No. 4
Nelson A, Don AR and Johnson JD (1993) Tobacco mosaic virus infection of transgenic Nicotiana tabacumplants is inhibited by antisense constructs directed at the 50 region of viral RNA. Gene 127: 227-232
Pałucha A, Sadowy E, Kujawa A, Juszczuk M., Zagórski W and Hulanicka D (1994) Nucleotide sequence of RNA of a Polish isolate of potato leafroll luteovirus Acta Bioch Polon 41: 405- 414
Powell PA, Stark DM, Sanders PR and Beachy RN (1989) Protection against tobaco mosaic virus in transgenic plants that express tobacco mosaic virus antisense RNA. Proc Natl Acad Sci USA 86: 6949-6952
Rezaian MA, Skene KGM and Ellis JG (1988) Anti-sense RNAs of cucumber mosaic virus in transgenic plants assessed for control of the virus. Plant Mol Biol 11: 463-471
Syller J (1985) Comparison of some isolates of potato leaf roll virus in Poland. Phytopathologische Zeitschrift 113: 17-23
van den Heuvel JFJ, de Blank CM, Peters D and van Lent JWM (1995) Localisation of potato leafroll virus in leaves of secondarily-infected potato plants. Eur J Plant Pathology 101: 567-571
van der Wilk F, Huisman M., Cornelissen BJC, Huttinga H and Gold-bach R (1989) Nucleotide sequence and organisation of potato leafroll virus genomic RNA. FEBS Lett 245: 51-56
van der Wilk F, Posthumus-Lutke Willink D, Huisman MJ, Huttinga H and Goldbach R (1991) Expression of the potato leafroll luteovirus coat protein gene in transgenic potato plants inhibits viral infection. Plant Mol Biol 17: 431-439
Verwoerd TC, Dekker BMM and Hoekema A (1989) A small-scale procedure for the rapid isolation of plant RNAs. Nucl Acid Res 17:2632
Zoeten GA (1991) Risk Assessment: Do we let history repeat itself? Phytopathology 81: 585-586
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pałucha, A., Zagórski, W., Chrzanowska, M. et al. An antisense coat protein gene confers immunity to potato leafroll virus in a genetically engineered potato. European Journal of Plant Pathology 104, 287–293 (1998). https://doi.org/10.1023/A:1008603302807
Issue Date:
DOI: https://doi.org/10.1023/A:1008603302807