Abstract
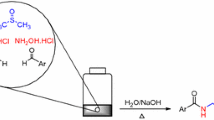
A novel four-component reaction approach to the efficient synthesis of triamide and amidodiester derivatives using amines or alcohols, aldehydes and alkyl or aryl isocyanides in the presence of Meldrum's acid as a CH acid, instead of carboxylic acid of Ugi four-component reaction, was studied.
Similar content being viewed by others
References
Dömling, A. and Ugi, I., Multicomponent reactions with isocyanides., Angew. Chem. Int. Ed., 39 (2000) 3168-3210.
Ugi, I., Lohberger, S. and Karl, R., in Comprehensive of organic synthesis; Trost, B. M., Fleming, I. and Heathcock, C. H., Eds., Pergamon: New York, 1991; Vol. 2, pp 1083-1109.
Ugi, I., The ?-addition of immonium ions and anions to isonitriles accompanied by secondary reactions, Angew. Chem. Int. Ed. Engl., 1 (1962) 8-21.
Keating, T. A. and Armstrong, R. W., The Ugi five-component condensation using CO2, CS2 and COS as oxidized carbon sources, J. Org. Chem., 63 (1998) 867-871.
Shaabani, A., Teimouri, M. B. and Bijanzadeh, H. R., One-pot three component condensation reaction in water: An efficient and improved procedure for the synthesis of furo[2,3-d]pyrimidine-2,4(1H,3H)-diones, Tetrahedron Lett., 43 (2002) 9151-9154.
Shaabani, A. and Teimouri, M. B., The reaction of alkyl isocyanides and benzylidene Meldrum's acid drivatives in the presence of water: A one-pot synthesis of 4-(alkylamino)-3-aryl-4-oxobutanoic acids, J. Chem. Research(S), (2002) 433-435.
Shaabani, A., Teimouri, M. B. and Bijanzadeh, H. R., The reaction of alkyl isocyanides and dialkylacetylene dicarboxylates with phthalic anhydride derivatives: A novel synthesis of ?-spiroiminolactones, J. Chem. Research(S), (2002) 381-383.
Shaabani, A., Bazgir, A., Soleimani, K. and Bijanzadeh, H. R., Reaction between alkyl isocyanides and 1,1,1,5,5,5-hexafluoropentane-2,4-dione in the presence of water: Onepot synthesis of highly fluorinated ?-dihydroxy-?-hydroxy amides and ?-keto-?-hydroxy amides, J. Flourine Chem., 116 (2002) 93-95.
Shaabani, A., Yavari, I., Teimouri, M. B., Bazgir, A. and Bijanzadeh, H. R., New and efficient synthesis of dialkyl 2-[1-p-nitrophenyl-2-(alkylamino)-2-oxoethyl]malonates, Tetrahedron, 57 (2001) 1375-1378.
Shaabani, A., Ajabi, S., Farrokhzad, F. and Bijanzadeh, H. R., [1+4] Cycloaddition of isocyanides with 2-acetyl-1,4-benzoquinone; A conveient synthesis of isobenzofuran-4,7-quinones, J. Chem. Research(S), (1999) 582-583.
Yavari, I., Shaabani, A. and Maghsoodlou, M. T., On the reaction between alkyl isocyanides and 3-benzylidene-2,4-pentanedione. A convenient synthetic route to densely functionalized furanes, Monatsh. Chem., 128 (1997) 697-700.
Shaabani, A. and Farrokhzad, F., [1 + 4] Cycloaddition of isocyanides with 3-(1-hydroxyethylidene)pentane-2,4-dione. A convenient synthesis of iminolactones, J. Chem. Research(S), (1997) 344.
McNab, H., Meldrum's acid, Chem. Soc. Rev., 7 (1978) 345-358.
Bigi, F., Carloni, S., Ferrari, L., Maggi, R., Mazzacani, A. and Sartori, G., Clean synthesis in water. Part 2: Uncatalysed condensation reaction of Meldrum's acid and aldehydes, Tetrahedron Lett., 42 (2001) 5203-5205.
Tietze, L. F. and Beifuss, U. W. E., in 'Comprehensive Organic Synthesis', Trost, B. M., Fleming, I., Heathcock, C. H., eds., Pergamon Press: Oxford, 2 (1991) 341-394.
Marchand, E., Morel, G. and Sinbandhit, S., A new access to 2-(alkylamino)-and 2-(arylamino)pyrroles by addition of isocyanides to protonated 1-azabutadienes, Eur. J. Org. Chem., (1999) 1729-1738.
Morel, G., Marchand, E., Sinbandhit, S. and Carlier, R., á-Thioxothioamides: a formal [1 + 4] cycloaddition reaction with isocyanides and diisocyanides and its application to a new straightforward formation of extended tetrathiofulvalenes, Eur. J. Org. Chem., (2001) 655-662.
Nair, V., Menon, R. S., Vinod, A. U. and Viji, S., A facile three-component reaction involving [1 + 4] cycloaddition leading to furan annulated heterocycles, Tetrahedron Lett., 43 (2002) 2293-2295.
Figueroa-Villar, J. D., Carneiro, C. L., Cruz, E. R., Synthesis of 6-phenylaminofuro[2,3-d]pyrimidine-2,4(1H,3H)-diones from barbiturylbenzylidens and isonitriles, Heterocycles, 34 (1992) 891-893.
Oikawa, Y., Sugano, K., and Yonemitsu, O., Meldrum's acid in organic synthsis. 2. A general and versatile synthesis of ?-ketoesters, J. Org. Chem., 43 (1978) 2087-2088.
Oikawa, Y., Hirasawa, H. and Yonemitsu, O., Meldrum's acid in organic synthesis. 1. A convenient one-pot synthesis of ethyl indolepropionates, Tetrahedron Lett., 19 (1978) 1759-1762.
Chen, B.-C., Meldrum's acid in organic synthesis, Heterocycles, 32 (1991) 529-597.
Davidson, D. and Bernhard, S. A., The structure of Meldrum's supposed ?-lactonic acid, J. Am. Chem. Soc., 70 (1948) 3426-3428.
Corey, E. J., The mechanism of decarboxylation of ?, ?-and ?, ?-unsaturated malonic acid derivatives and the course of decarboxylative condensation reactions in pyridine, J. Am. Chem. Soc., 74 (1952) 5897-5905.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shaabani, A., Teimouri, M.B., Bazgir, A. et al. Introducing a novel class of four-component reactions. Mol Divers 6, 199–206 (2003). https://doi.org/10.1023/B:MODI.0000006759.20910.f1
Issue Date:
DOI: https://doi.org/10.1023/B:MODI.0000006759.20910.f1