Abstract
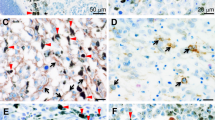
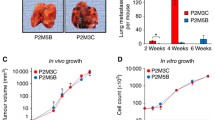
In the present study, we examined the autocrine/paracrine role of IL-8 in melanoma growth and metastasis by analyzing the expression and functional significance of IL-8 receptors, CXCR1 and CXCR2 in human malignant melanoma cells with different metastatic potential. CXCR1 and CXCR2 mRNA and protein levels were analyzed by reverse trannscriptase-based polymerase chain reaction, immunohistochemistry, immunoprecipitation, flow cytometry and ligand binding assay in melanoma cells in vitro and xenografted in nude mice. Melanoma cells constitutively expressed CXCR1 and CXCR2 mRNA and protein. Highly metastatic A375SM cells expressed higher levels of CXCR1 and CXCR2 mRNA and protein in vitro and in vivo as compared to low metastatic A375P and non-metastatic SBC-2 melanoma cells. Treatment of SBC-2 and A375P cells with exogenously added recombinant IL-8 significantly enhanced their proliferation and invasive potential. Further neutralizing antibodies to CXCR1 and CXCR2 inhibited proliferation and invasive potential of unstimulated and IL-8-stimulated A375P cells. In summary, the data suggest that constitutive expression of CXCR1 and CXCR2 play an important role regulating the IL-8-mediated metastatic phenotype in human malignant melanoma cells.
Similar content being viewed by others
References
Lee JE. Factors associated with melanoma incidence and prognosis. Semin Surg Oncol 1996; 12: 379–85.
Clark WH, Elder DE, Guerry D et al. A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma. Hum Pathol 1984; 15: 1147–65.
Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1996. CA Cancer J Clin 1996; 46: 5–27.
Jemal A, Murray T, Samuels A et al. Cancer statistics, 2003. CA Cancer J Clin 2003; 53: 5–26.
Herlyn M. Human melanoma: Development and progression. Cancer Metast Rev 1990; 9: 101–112.
Kerbel RS. Growth dominance of the metastatic cancer cell: Cellular and molecular aspects. Adv Cancer Res 1990; 55: 87–132.
Fidler IJ and Radinsky R. Search for genes that suppress cancer metastasis. J Natl Cancer Inst 1996; 88: 1700–03.
Herlyn D, Iliopoulos D, Jensen PJ et al. In vitro properties of human melanoma cells metastatic in nude mice. Cancer Res 1990; 50: 2296–302.
Herlyn M, Kath R, WIlliams N et al. Growth-regulatory factors for normal, premalignant, and malignant human cells in vitro. Adv Cancer Res 1990; 54: 213–34.
Lazar-Molnar E, Hegyesi H, Toth S, Falus A. Autocrine and paracrine regulation by cytokines and growth factors in melanoma. Cytokine 2000; 12: 547–54.
Herlyn M. Human melanoma: Development and progression. Cancer Metast 1990; 9: 101–9.
Singh RK, Gutman M, Radinsky R. Heterogeneity of cytokine and growth factor gene expression in human melanoma cells with different metastatic potentials. J Interferon Cytokine Res 1995; 15: 81–7.
Singh RK, Gutman M, Radinsky R et al. Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res 1994; 54: 3242–7.
Singh RK, Varney ML, Bucana CD, Johansson SL. Expression of interleukin-8 in primary and metastatic malignant melanoma of the skin. Melanoma Res 1999; 9: 383–7.
Schadendorf D, Moller A, Algermissen B et al. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J Immunol 1993; 151: 2667–75.
Koch AE, Polverini PJ, Kunkel SL et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992; 258: 1798–801.
Strieter RM, Kunkel SL, Elner VM et al. Interleukin-8. A corneal factor that induces neovascularization. Am J Pathol 1992; 141: 1279–84.
Wang JM, Taraboletti G, Matsushima K et al. Induction of haptotactic migration of melanoma cells by neutrophil activating protein/interleukin-8. Biochem Biophys Res Commun 1990; 169: 165–70.
Oppenheim JJ, Zachariae CO, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol 1991; 9: 617–48.
Miller MD, Krangel MS. Biology and biochemistry of the chemokines: A family of chemotactic and inflammatory cytokines. Crit Rev Immunol 1992; 12: 17–46.
Matsushima K, Oppenheim JJ. Interleukin 8 and MCAF: Novel in-flammatory cytokines inducible by IL 1 and TNF. Cytokine 1989; 1: 2–13.
Moser B, Barella L, Mattei S et al. Expression of transcripts for two interleukin 8 receptors in human phagocytes, lymphocytes and melanoma cells. Biochem J 1993; 294 (Pt 1): 285–92.
Holmes WE, Lee J, Kuang WJ, et al. Structure and functional expression of a human interleukin-8 receptor. Science 1991; 253: 1278–80.
Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science 1991; 253: 1280–3.
Horuk R, Chitnis CE, Darbonne WC et al. A receptor for the malarial parasite Plasmodium vivax: The erythrocyte chemokine receptor. Science 1993; 261: 1182–4.
Moser B and Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol 2001; 2: 123–8.
Peiper SC, Wang ZX, Neote K et al. The Duffy antigen/receptor for chemokines (DARC) is expressed in endothelial cells of Duffy negative individuals who lack the erythrocyte receptor. J Exp Med 1995; 181: 1311–7.
Addison CL, Daniel TO, Burdick MD et al. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokineinduced angiogenic activity. J Immunol 2000; 165: 5269–77.
Kozlowski JM, Hart IR, Fidler IJ, Hanna N. A human melanoma line heterogeneous with respect to metastatic capacity in athymic nude mice. J Natl Cancer Inst 1984; 72: 913–7.
Verschraegen CF, Giovanella BC, Mendoza JT et al. Specific organ metastases of human melanoma cells injected into the arterial circulation of nude mice. Anticancer Res 1991; 11: 529–35.
Li A, Dubey S, Varney ML et al. IL-8 Directly Enhanced Endothelial Cell Survival, Proliferation, and Matrix Metalloproteinases Production and Regulated Angiogenesis. 2003; 170: 3369–76.
Singh RK, Llansa N, Bucana CD et al. Cell density-dependent regulation of basic fibroblast growth factor expression in human renal cell carcinoma cells. Cell Growth Differ 1996; 7: 397–404.
Singh RK, Ino K, Varney M et al. Immunoregulatory cytokines in bone marrow and peripheral blood stem cell products. Bone Marrow Transplant 1999; 23: 53–62.
Singh RK, Bucana CD, Gutman M et al. Organ site-dependent expression of basic fibroblast growth factor in human renal cell carcinoma cells. Am J Pathol 1994; 145: 365–74.
Goel A, Colcher D, Baranowska-Kortylewicz J et al. Genetically engineered tetravalent single-chain Fv of the pancarcinoma monoclonal antibody CC49: Improved biodistribution and potential for therapeutic application. Cancer Res 2000; 60: 6964.
Singh RK, Varney ML. Regulation of interleukin 8 expression in human malignant melanoma cells. Cancer Res 1998; 58: 1532–7.
Hendrix MJ, Seftor EA, Seftor RE, Fidler I. JA simple quantitative assay for studying the invasive potential of high and low human metastatic variants. Cancer Lett 1987; 38: 137–47.
Chu YW, Runyan RB, Oshima RG, Hendrix MJ.Expression of complete keratin filaments in mouse L cells augments cell migration and invasion. Proc Natl Acad Sci USA 1993; 90: 4261–65.
Li A, Varney ML, Singh RK. Expression of interleukin 8 and its receptors in human colon carcinoma cells with different metastatic potentials. Clin Cancer Res 2001; 7: 3298–304.
Li A, Dubey S, Varney ML, Singh RK. Interleukin-8-Induced Proliferation, Survival, and MMP Production in CXCR1 and CXCR2 Expressing Human Umbilical Vein Endothelial Cells. Microvasc Res 2002; 64: 476–81.
Singh RK, Varney ML. IL-8 expression in malignant melanoma: Implication in growth and metastasis. Histol Histopathol 2000; 15: 843–9.
Luca M, Huang S, Gershenwald JE et al. Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. Am J Pathol 1997; 151: 1105–13.
Huang S, Mills L, Mian B et al. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am J Pathol 2002; 161: 125–34.
Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol 2001; 19: 577–83.
Nurnberg W, Tobias D, Henz BM et al. Authors' reply. J Pathol 2000; 192: 414–5.
Scheibenbogen C, Mohler T, Haefele J, et al. Serum interleukin-8 (IL-8) is elevated in patients with metastatic melanoma and correlates with tumour load. Melanoma Res. 1995; 5: 179–81.
Nurnberg W, Tobias D, Otto F et al. Expression of interleukin-8 detected by in situ hybridization correlates with worse prognosis in primary cutaneous melanoma. J Pathol 1999; 189: 546–51.
Singh RK, Varney ML. IL-8 expression in malignant melanoma: Implications in growth and metastasis. Histol Histopathol 2000; 15: 843–9.
Norgauer J, Metzner B, Schraufstatter I. Expression and growthpromoting function of the IL-8 receptor beta in human melanoma cells. J Immunol 1996; 156: 1132–7.
Luque I and Gelinas C. Rel/NF-kappa B and I kappa B factors in oncogenesis. Semin Cancer Biol 1997; 8: 103–11.
Satyamoorthy K, Li G, Vaidya B, et al. Insulin-like growth factor-I-induced migration of melanoma cells is mediated by interleukin-8 induction. Cell Growth Differ 2002; 13: 87–93.
Smith DR, Polverini PJ, Kunkel SL, et al. Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma. J Exp Med 1994; 179: 1409–15.
Fujisawa N, Hayashi S, Kurdowska A et al. Inhibition of GROalphainduced human endothelial cell proliferation by the alpha-chemokine inhibitor antileukinate. Cytokine 1999; 11: 231–38.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Varney, M.L., Li, A., Dave, B.J. et al. Expression of CXCR1 and CXCR2 receptors in malignant melanoma with different metastatic potential and their role in interleukin-8 (CXCL-8)-mediated modulation of metastatic phenotype. Clin Exp Metastasis 20, 723–731 (2003). https://doi.org/10.1023/B:CLIN.0000006814.48627.bd
Issue Date:
DOI: https://doi.org/10.1023/B:CLIN.0000006814.48627.bd