Abstract
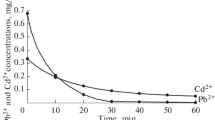
Adsorption of Zn2+ and Cd2+ ions from aqueous waste solutions on iron(III) titanate as inorganic ion exchange material was investigated to determine the effect of contact time, pH of solution and the reaction temperatures. Batch kinetic studies were carried out and showed that the time of equilibrium for both Zn2+ and Cd2+ ions was attained within three hours, and the order of kinetic reaction is the first order reaction. Batch distribution coefficients of Zn2+ and Cd2+ ions on iron(III) titanate as a function of pH have been studied at 25, 40 and 60 ± 1°C. From the obtained results we found that the K d values decreased with increasing reaction temperatures. Enthalpy change (ΔH) values for Zn2+ and Cd2+ ions were found to be −8.19 and −22.49 kJ/mol, respectively. The data of adsorption of Zn2+ and Cd2+ ions at various concentrations were fitted with the Freundlich isotherm. Finally, separation of the above mentioned cations on iron(III) titanate in a column was performed.
Similar content being viewed by others
References
Abe, M., P. Wang, R. Chitrakar, and M. Tsuji, Analyst, 114, 435 (1989).
Abou-Mesalam, M.M. and I.M. El-Naggar, Arab Journal of Nuclear Science and Applications, 35(1), 45 (2002).
Clark, A., Theory of Adsorption and Catalysis, Academic Press, New York 1970, p. 54.
De, A.K. and P. Chakraborty, Separation Science and Technology, 17(9), 1129 (1982).
El-Naggar, I.M., M.M. Abou-Mesalam, M.M. Abdel-Hamid, and S.A. Shady, in 7th Conference of Nuclear Science and Applications, 6-10 Feb, Cairo, Egypt, 2000.
El-Naggar, I.M., E.S. Zakaria, M.M. Abou-Mesalam, and H.F. Aly, Czechoslovak Journal of Physics, 49 (Suppl. S1), (1999).
El-Naggar, I.M., M.M. Abou-Mesalam, and S.A. Shady, in 7th Conference of Nuclear Science and Applications, 6-10 Feb., Cairo, Egypt, 2000.
Helffrich, F., Ion Exchange, McGraw Hill, New York, 1962.
Lienonen, H., J. Lehto, and A. Marela, Reactive Polymers, 23, 221 (1994).
Mishra, S.P., and N. Srinivasu, Indian Journal of Technology, 30, 409 (1992).
Oliverira, S.F. and C. Airoldi, Mikrochim. Acta, 110, 95 (1993).
Qureshi, M. and K.G. Varshney, Inorganic Ion Exchangers in Chemical Analysis, CRC Press, Boca Raton, Florida, 1991.
Weber, W.J. and J.C. Morris, Journal of Sanitary Engineering Division, ASCE, SA2, 31 (1963).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abou-Mesalam, M. Applications of Inorganic Ion Exchangers: II—Adsorption of Some Heavy Metal Ions from Their Aqueous Waste Solution Using Synthetic Iron(III) Titanate. Adsorption 10, 87–92 (2004). https://doi.org/10.1023/B:ADSO.0000024038.32712.18
Issue Date:
DOI: https://doi.org/10.1023/B:ADSO.0000024038.32712.18