Abstract
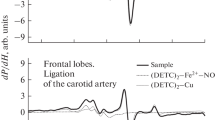
Previous studies have shown that brain tissue hypoxia results in increased N-methyl-D-aspartate (NMDA) receptor activation and receptor-mediated increase in intracellular calcium which may activate Ca++-dependent nitric oxide synthase (NOS). The present study tested the hypothesis that tissue hypoxia will induce generation of nitric oxide (NO) free radicals in cerebral cortex of newborn guinea pigs. Nitric oxide free radical generation was assayed by electron spin resonance (ESR) spectroscopy. Ten newborn guinea pigs were assigned to either normoxic (FiO2 = 21%, n = 5) or hypoxic (FiO2 = 7%, n = 5) groups. Prior to exposure, animals were injected subcutaneously with the spin trapping agents diethyldithiocarbamate (DETC, 400 mg/kg), FeSO4.7H2O (40 mg/kg) and sodium citrate (200mg/kg). Pretreated animals were exposed to either 21% or 7% oxygen for 60 min. Cortical tissue was obtained, homogenized and the spin adducts extracted. The difference of spectra between 2.047 and 2.027 gauss represents production of NO free radical. In hypoxic animals, there was a difference (16.75 ± 1.70 mm/g dry brain tissue) between the spectra of NO spin adducts identifying a significant increase in NO free radical production. In the normoxic animals, however, there was no difference between the two spectra. We conclude that hypoxia results in Ca2+- dependent NOS mediated increase in NO free radical production in the cerebral cortex of newborn guinea pigs. Since NO free radicals produce peroxynitrite in presence of superoxide radicals that are abundant in the hypoxic tissue, we speculate that hypoxia-induced generation of NO free radical will lead to nitration of a number of cerebral proteins including the NMDA receptor, a potential mechanism of hypoxia-induced modification of the NMDA receptor resulting in neuronal injury.
Similar content being viewed by others

REFERENCES
Moncada, S., Palmer, R. M. J., and Higgs, E. A. 1991. Nitric oxide: physiology, pathophysiology and pharmacology. Parmacol. Rev. 43:109–134.
Schuman, E. M. and Madison, D. V. 1994. Nitric oxide and synaptic function. Ann Rev. Neurosci. 17:153–183.
Radi, R., Beckman, J. S., and Freeman, B. A. 1991. Peroxynitrite-induced membrane lipid oxidation, the cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 266:4244–4250.
Nowicki, J. P., Duval, D., Poignet, H., and Scatton, B. 1991. Nitric oxide mediates neuronal death after focal cerebral ischemia in the mouse. Eur. J. Pharmacol. 204:339–340.
Rubbo, H., Radi, R., Trujillo, M., Telleri R., Kalyanraman, B., Barnes, S., Kerk, M., and Freeman, B. A. 1994. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. J. Biol. Chem. 269:26066–260759.
Vidwans, A. S., Kim, S., Coffin, D. O., Wink, D. A., and Hewett, S. J. 1999. Analysis of the neuroprotective effect os of various nitric oxide donor compounds in murine cortical cell culture. J. Neurochem. 72:1843–1852.
Numagami, Y., Zubrow, A. B., Mishra, O. P., and Delivoria-Papadopoulos, M. 1997. Lipid free radical generation and brain cell membrane alteration following nitric oxide synthase inhibition during cerebral hypoxia in the newborn piglet. J. Neurochem. 69:1542–1547.
Bredt, D. S. and Snyder, S. H. 1989. Nitric oxide mediates glutamate linked enhancement of cGMP levels in cerebellum. Proc. Natl. Acad. Sci. USA 86:9030–9033.
Garthwaite, J., Garthwaite, G., Palmer, R. M., and Moncada, S. 1989. NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur. J. Pharmacol. 172:413–416.
Frandsen, A. and Schousboe, A. 1993. Excitatory amino acid mediated cytotoxicity and calcium homeostasis incultured neurons. J. Neurochem. 60:1202–1211.
Lipton, S. A. and Rosenberg, P. A. 1994. Excitatory amino acids as a final common pathway for neurologic disorders. New Engl. J. Med. 330:613–622.
Mishra, O. P. and Delivoria-Papadopoulos, M. 1992. NMDA receptor modification of the fetal guinea pig brain during hypoxia. Neurochem. Res. 17:1211–1216.
Hoffman, D. J., DiGiacomo, J. E., Marro, P. J., Mishra, O. P., and Delivoria-Papadopoulos, M. 1994. Hypoxia-induced modification of the N-methyl-D-aspartate (NMDA) receptor in the brain of newborn piglets. Neurosci. Lett., 167:156–160.
Razdan, B., Kubin, J. A., Mishra, O. P., and Delivoria-Papadopoulos, M. 1996. Modification of the glycine (co-activator) binding site of the N-methyl-D-aspartate receptor in the guinea pig fetus brain during development following hypoxia. Brain Res. 733:15–20.
Fritz, K. Fritz, K. I., Mishra, O. P., and Delivoria-Papadopoulos, M. 1999. Mg++-modification of the NMDA receptor during graded hypoxia in cerebral cortex of newborn piglets. Neurosci. 92:685–692.
Zanelli, S. A., Numagami, Y., McGowan, J. E., Mishra, O. P., and Delivoria-Papadopoulos, M. 1999. NMDA receptor mediated calcium influx in cerebral cortical synaptosomes of the hypoxic guinea pig fetus. Neurochem. Res. 24:437–446.
Tominaga, T., Sato, S., Ohnishi, T., and Ohnishi, S. 1994. Electron paramagnetic resonance (EPR) detection of nitric oxide produced durin forebrain ischemia of the rat. J. Cereb. Blood Flow Metab. 14:715–722.
Lamprecht W., Stein P., Heinz F., and Weisser, H. 1994. Creatine Phosphate. In: Methods of Enzymatic Analysis (Bergmeyer HU ed.), Vol. 4, Academic Press: New York, pp 1777–1781.
Forstermann, U., Boissel, J. P., and Kleinert, H. 1998. Expressional control of the 'constitutive' isoforms of nitric oxide synthase (NOS I and NOS III). FASEB J., 12:773–790.
Shaul, P. W., North, A. J., Brannon, T. S., Ujiie, K., Wells, L. B., Nisen, P. A., Lowenstein, C. J., Snyder, S. H., and Star, R. A. 1995. Prolonged in vivo hypoxia enhances nitric oxide synthase type I and type III gene expression in adult rat lung. Am. J. Respir. Cell. Mol. Biol., 13:167–174.
Prabhakar, N. R., Rao, S., Premkumar, D., Pieramici, S. F., Kumar, G. K., and Kalaria, R. K. 1996. Regulation of neuronal nitric oxide synthase gene expression by hypoxia. Role of nitric oxide in respiratory adaptation to low PO2. Adv. Exp. Med. Biol. 410:345–348.
Guo, Y., Ward, M. E., Beasjours, S., Mori, M., and Hussain, S. N. A. 1997. Regulation of cerebellar nitric oxide production in response to prolonged in vivo hypoxia. J. Neurosci. Res. 49:89–97.
Zhang, Z. G., Chopp, M., Gautam, S., Zaloga, C., Zhang, R. L., Schmidt, H. H., Pollock, J. S., and Forstermann, U. 1994. Upregulation of neuronal nitric oxide synthase and mRNA, and selective sparing of nitric oxide synthase-containing neurons after focal cerebral ischemia in rat. Brain Res. 654:85–95.
Samdani, A. F., Dawson, T. M., and Dawson, V. L. 1997. Nitric oxide synthase in models of focal ischemia. Stroke 28: 1283–1288.
Mishra, O. P. and Delivoria-Papadopoulos, M. 1999. Cellular mechanisms of hypoxic injury in the developing brain. Brain Res. Bull. 48:233–238.
Choi, D. W. 1995. Calcium: Still center-stage in hypoxicischemic neuronal death. TINS 18:58–60.
Kristian, T. and Siesjo, B. K. 1998. Calcium in ischemic cell death. Stroke 29:705–718.
Smith, D. S., Rosenthal, M., Nioka, S., Subramanian, H., and Chance, B. 1986. Brain cytochromes and change in energy states. Soc. Magn. Res. Abstr. 4:1113–1114.
Turrens, J. G., Alexandre, A., and Lehninger, A. L. 1985. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch. Biochem. Biophys. 237:271–278.
Choi, D. W. 1990. Cerebral hypoxia: some new approaches and unanswered questions. J. Neurosci. 10:2493–2501.
Rothman, S. M. and Olney, J. W. 1986. Glutamate and the pathophysiology of hypoxic-ischemic brain damage. Ann. Neurol. 19:105–111.
Monoghan, D. T., Bridges, R. J., and Cotman, C. W. 1989. The excitatory amino acid receptors: Their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 29:365–402.
Simon, R. P., Swan, J. H., Griffiths, T., and Meldrum, B. S. 1984. Blockade of N-methyl-D-aspartate receptors may protect against ischaemic damage in the brain. Science 226:850–852.
Kochnar, A., Zivan, J. A., Lyden, P. D., and Mazzarella, V. 1988. Glutamate antagonist therapy reduced neurologic deficits produced by focal central nervous system ischemia. Arch. Neurol. 45:148–153.
Park, C. K., Nehis, D. G., Graham, D. I., Teasdale, G. M., and McCulloch, J. 1988. The glutamate antagonist MK-801 reduces focal ischemic brain damage in the rat. Ann. Neurol. 24: 543–551.
Bullock, R., Graham, D. I., Min-Hsiung, C., Lowe, D., and Mc-Culloch, J. 1990. Focal cerebral ischemia in the cat: pretreatment with a competitive NMDA receptor antagonist, D-CPPene. J. Cereb. Blood Flow and Metab. 10:668–674.
Tacconi, S., Ratti, E., Marien, M. R., Gaviraghi, G., and Bowery, N. G. 1993. Inhibition of [3H]-(+)-MK-801 binding to rat brain sections by CPP and 7-chlorokyneuric acid: an autoradiographic analysis. Br. J. Pharmacol. 108:668–674.
Zeevalk, G. D. and Nicklas, W. J. 1992. Developmental differences in antagonism of NMDA toxicity by the polyamine site antagonist ifenprodil. Dev. Brain Res. 65:147–155.
Hoffman, D. J., Marro, P. J., McGowan, J. E., Mishra, O. P., and Delivoria-Papadopoulos, M. 1994. Protective effect of MgSO4 infusion on NMDA receptor binding characteristics during cerebral cortical hypoxic in the newborn piglet. Brain Res. 644:144–149.
McDonald, J. W., Silverstein, F. S., Cardona, D., Hudson, C., Chen, R., and Johnston, M. V. 1990. Systemic administration of MK-801 protects against N-methyl-D-aspartate and quisquilate-mediated neurotoxicity in perinatal rats. Neurosci. 36: 589–599.
Dawson, V. L., Dawson, T. M., London, E. D., Bredt. D. S., and Snyder, S. H. 1991. Nitric oxide mediates glutamate neurotoxicity in primary cortical culture. Proc. Natl. Acad. Sci. USA 88:6368–6371.
Dawson, T. M., Zhang, J., Dawson, V. L., and Snyder, S. H. 1994. Nitric oxidecellular regulation and neuronal injury. Prog. Brain Res. 103:365–369.
Huang, J., Huang, P. L., Panathian, N., Dalkara, T., Fishman, M. C., and Moskowitz, M. A. 1994. Effect of cerebral ischemia in mice deficient neuronal nitirc oxide synthase. Science 265: 1883–1885.
Yun, H.-Y., Dawson, V. L., and Dawson T. M. 1997. Nitric oxide in health and diseases of the nervous system. Mol. Psychiatr. 2:300–310.
Bhat, G. K., Mahesh, V. B., Lamar, C. A., Ping, L., Aguan, K., and Brann, D. W. 1997. Histochemical localization of nitric oxide neurons in the hypothalamus: association with gonadotropinrelaeasing hormone neurons and co-localization with N-methyl-D-aspartate receptors. Neuroendocrinology 62:187–197.
Aoki, C., Rhee, J., Lubin, M., and Dawson, T. M. 1997. NMDA-R1 subunit of the cerebral cortex co-localizes with neuronal nitric oxide synthase at pre-and postsynaptic sites and in spines. Brain Res. 750:25–40.
Bredt, D. S. and Snyder, S. H. 1990. Isolation of nitric oxide synthase, a calmodulin-requiring enzyme. Proc. Natl. Acd. Sci. 87:682–685.
Garthwaite, J. Charles, S. L., and Chess-William, R. 1988. Endothelium derived relaxing factorrelease on activation of NMDA receptor suggests roles oas intracellular messenger in brain. Nature 336:385–388.
Kiedrowski, L. Costa, E. and Wroblewski, J. T. 1992. Glutamate receptor agonists stimulate nitric oxide synthasein primary culture of cerebellar granule cells. J. Neurchem. 58:335–341.
Aoki, C., Fenstemaker, S., Lubin, M., and Go, C. G. 1993. Nitric oxide synthase in visual cortex of monocular monkey as revealed by light and electron microscopic immunochemistry. Brain Res. 620:97–113.
Christopherson, K. S., Hiller, B. S., Lim, W. A., and Bredt, D. S. 1999. PSD-95assembles a ternary complex with the N-methyl-D-aspartate receptor and a bivalent neuronal NO synthase PDZ domain. J. Biol. Chem. 274:27467–27473.
Sattler, R., Xion, Z., Lu, W.-Y., Hafner, M., MacDonald, J. F., and Tymianski, M. 1999. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science 284:1845–1848.
Beckman, J. S., Beckman, T. W., Chen, J., Marshall, P. A., and Freeman, B. A. 1980. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci., 87:1620–1624.
Ischiropoulos, H., Zhu, L., and Beckman, J. S. 1992. Peroxynitrite formation from macrophage-derived nitric oxide. Arch. Biochem. Biophys. 298:446–451.
Lipton, S. A., Choi, Y. B., Pan, Z. H., Lei, S. Z., Chen, H. S., Sucher, N. J., Loscalzo, J., Singel, D. J., and Stamler, J. S. 1993. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature 364:626–632.
Radi, R., Beckman, J. S., Bush, K. M., and Freeman, B. A. 1991. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 266:4244–4250.
Ischiropoulos, H., Zhu, L., Chen, J., Tsai, M., Martin, J-C., Smith, C. D. and Beckman, J. S. 1992. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch. Biochem. Biophys. 298:431–437.
Beckman, J. S., Ischiropoulos, H., Zhu, I., van der Woerd, M., Smith, C. D., Chen, J., Harrison, J., Martin, J.-C., and Tsai, M. 1992. Kinetics of superoxide dismustase and iron-catalyzed nitration of phenolics by peroxynitrite. Arch. Biochem. Biophys. 298:438–445.
Beckman, J. S., Ye, Y. Z., and Anderson, P. G. 1994. Excessive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol. Chem. Hoppe-Seyler 375:81–88.
Yun, H. Y., Dawson, V. L., and Dawson, T. M. 1997. Nitric oxide in health and disease of the nervous system. Mol. Psych. 2:300–310.
Good, P. F., Hsu, A., Werner, P., Derl, D. P., and Warren, O. 1998. Protein nitration in Parkinson's Disease. J. Neuropath. Exper. Neurol. 57:338–342.
Iadecola, C. 1997. Bright and dark sides of nitric oxide in ischemic brain injury. TINS 20:132–139.
Beckman, J. S. Peroxynitrite, superoxide dismutase, and tyrosine nitration in neurodegeneration. Prog. Brain Res., 103: 271-280.
Beckman, J. S., Carson, M., Smith, C. D., and Koppenol, W. H. 1993. ALS, SOD and peroxynitrite. Nature 364-584.
Good, P. F., Werner, P., Hsu, A., Olanow, C. W., and Perl, D. P. 1996. Evidence for neuronal oxidative damage in Alzheimer's disease. Am. J. Pathol. 149:21–28.
Bruijn, L. I., Beal, M. F., and Becher, M. W. 1997. Elevated free nitrotyrosine levels, but not protein-bound nitrotyrosine of hydroxyl radicals, throughout amyotrophic lateral sclerosis (ALS)-like disease implicate tyrosine nitration as an aberrant in vivo property of one familial ALS-linked superoxide dismutase 1 mutant. Proc. Natl. Acad. Sci. USA 94:7606–7611.
Smith, M. A., Richey-Harris, P. L., Sayre, L. M., Beckman, J. S., and Perry, G. 1997. Widespread peroxynitrite-mediated damage in Alzheimer's disease. J. Neurosci. 17:2653–2657.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mishra, O.P., Zanelli, S., Ohnishi, S.T. et al. Hypoxia-Induced Generation of Nitric Oxide Free Radicals in Cerebral Cortex of Newborn Guinea Pigs. Neurochem Res 25, 1559–1565 (2000). https://doi.org/10.1023/A:1026610301978
Issue Date:
DOI: https://doi.org/10.1023/A:1026610301978