Abstract
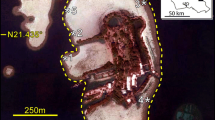
A large population of a previously unreported jellyfish occurred across the northern Gulf of Mexico (USA) from May through September of 2000. The jellyfish, identified as Phyllorhiza punctata by von Lendenfeld (1884), is not indigenous to the Gulf of Mexico or to the Atlantic Basin. Current theory states that this invasive species was introduced into the Atlantic from the Pacific Ocean through the Panama Canal about 45 years ago, and several confirmed reports indicated that a cryptic population may have existed in the northern Gulf since 1993. However, mesoscale hydrographic anomalies in spring 2000 may have directly transported the population from the Caribbean Sea where populations of P. punctata have been reported previously in Puerto Rico. We undertook a rapid-response sampling program from June through September to obtain ecological information regarding P. punctata in the highly productive northern Gulf waters. Jellyfish bell diameter increased by about 50% (from an average of 32 ± 12 to 45 ± 6 cm) as animals invaded nearshore waters during July. As the summer progressed, a net westerly distribution shift followed nearshore currents to an accumulation point near the mouth of Lake Borgne, Louisiana, in the far western Mississippi Sound. In the Lake Borgne aggregation, we estimated 5.37 × 106 medusae over an area of about 150 km2. All of the medusae appeared to lack the symbiotic algae that are present in all other described populations, and therefore must have depended solely on planktivory for their nutrition. Clearance rates estimated from ambient zooplankton, gut contents and published digestion times ranged from < 1 m3 d−1 for adult copepods to over 90 m3 d−1 for fish eggs. Based on these clearance rates, the central core of this aggregation (30 km2) was being turned over at least once per day with even higher turn-over in very concentrated `super-swarms'. Clogging of shrimp nets was the greatest economic impact, and perhaps contributed to millions of dollars in economic losses. The indirect effect of predation on eggs and larvae of commercially important finfish and shellfish remained intangible in the determination of economic effects. In 2001, P. punctata has recurred along southern Louisiana and has apparently spread into the coastal and lagoonal waters of the Florida east coast.
Similar content being viewed by others
References
Ari MN (1997) A Functional Biology of Scyphozoa. Chapman and Hall, New York, 316 pp
Carlton JT (1996) Pattern, process, and prediction in marine invasion ecology. Biological Conservation 78: 97–106
Carlton JT and Geller JB (1993) Ecological roulette: the global transport of non-indigenous marine organisms. Science 261: 78–82
Clarke TA and Abey GS (1998) The use of small mid-water attraction devices for investigation of the pelagic juveniles of carangid fishes in Kaneohe Bay, Hawaii. Bulletin of Marine Science 62(3): 947–955
Costello JH and Colin SP (1994) Morphology, fluid motion and predation by the scyphomedusa Aurelia aurita. Marine Biology 121: 327–334
Costello JH and Colin SP (1995) Flow and feeding by swimming scyphomedusae. Marine Biology 124: 399–406
Cowan Jr JH and Houde ED (1992) Size-dependent predation on marine fish larvae by ctenophores, scyphomedusae, and planktivorous fish. Fisheries and Oceanography 1(2): 113–126
Cutress CE (1971) Phyllorhiza punctata in the tropical Atlantic. Proceedings of the Association of Island Marine Laboratories in the Caribbean 9: 14
Dagg MJ (1995) Ingestion of phytoplankton by the micro-and mesozooplankton communities in a productive subtropical estuary. Journal of Plankton Research 17: 845–857
D'Ambra I, Costello JH and Bentivegna F (2001) Flow and prey capture by the scyphomedusa Phyllorhiza punctata von Lendenfeld 1884. Hydrobiologia 451: 223–227
da Silveira FL and Cornelius PFS (2000) New observations on medusae (Cnidaria, Scyphozoa, Rhizostomae) from northeast and south Brazil. Acta Biologica Leopoldensia 22: 9–18
deLafontaine Y and Leggett WC (1988) Predation by jellyfish on larval fish: an experimental evaluation employing in situ enclosures. Canadian Journal of Fisheries Aquatic Science 45: 1173–1190
Devaney, DM and Eldredge LG (1977) Reef and shore fauna of Hawaii. Section 1: Protozoa through Ctenophora. Bishop Museum Special Publication 64: 1–277
Fancett MS (1988) Diet and prey selectivity of scyphomedusae from Port Phillip Bay. Australia, Marine Biology 98: 503–509
Galil BS, Spanier E and Ferguson WW (1990) and The scyphomedusae of the Mediterranean coast of Israel, including two Lesepsian migrants new to the Mediterranean. Zoologische Medelingen 64: 95–105
Garcia JR (1990) Population dynamics and production of Phyllorhiza punctata (Cnidaria: Scyphozoa) in Laguna Joyuda, Puerto Rico. Marine Ecology Progress Series 64: 243–251
Garcia JR and Durbin E (1993) Zooplanktivorous predation by large scyphomedusae Phyllorhiza punctata (Cnidaria: Scyphozoa) in Laguna Joyuda. Journal of Experimental Marine Biology and Ecology 173: 71–93
Graham WM (1998) First report of Carybdea alata var. grandis (Reynaud 1830) (Cnidaria: Cubozoa) from the Gulf of Mexico. Science 16: 28–30
Graham WM (2001) Numerical increases and distributional shifts of Chrysaora quinquecirrha Desor and Aurelia aurita Linné (Cnidaria: Scyphozoa) in the northern Gulf of Mexico. Hydrobiologia 451: 97–111
Graham WM and Kroutil RM (2001) Size-based prey selectivity and dietary shifts in the jellyfish, Aurelia aurita. Journal of Plankton Research 23: 67–74
Graham WM, Pagès F and Hamner WM (2001) A physical context for gelatinous zooplankton aggregations: a review. Hydrobiologia 451: 199–212
Heeger T, Piatkowski U and Moeller H (1992) Predation on jellyfish by the cephalopod Argonauta argo. Marine Ecology Progress Series 88: 293–296
Hsieh Y, Leong F and Barnes KW (1996) Inorganic constituents in fresh and processed cannonball jellyfish (Stomolophus meleagris) Journal of Agriculture and Food Chemistry 44: 3117–3119
Ishii H and Baamstedt U (1998) Food regulation of growth and maturation in a natural population of Aurelia aurita (L.). Journal of Plankton Research 20(5): 805–816
Ivanov VP, Kamakin AM, Ushivtzev VB, Shiganova T, Zhukova O, Aladin N, Wilson SI, Harbison GR and Dumont HJ (2000) Invasion of the Caspian Sea by the comb jellyfish Mnemiopsis leidyi (Ctenophora). Biological Invasions 2: 255–258
Kideys AE (1994) Recent dramatic changes in the Black Sea ecosystem: the reason for the sharp decline in Turkish anchovy fisheries. Journal of Marine Systems 5: 171–181
Kingsford MJ, Pitt KA and Gillanders BM (2000) Management of jellyfish fisheries, with special reference to the Order Rhizostomeae. Oceanography and Marine Biology Annual Reviews 38: 85–156
Kramp PL (1961) Synopsis of the medusae of the world. Journal of the Marine Biological Associations, UK 40: 1–469
Kramp PL (1970) Zoogeographical studies on Rhizostomeae (Scyphozoa). Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening 133: 7–30
Larson RJ (1986) Water content, organic content, and carbon and nitrogen composition of medusae from the northeast Pacific. Journal of Experimental Marine Biology and Ecology 99: 107–120
Larson RJ (1991) Diet, prey selection and daily ration of Stomolophus meleagris, a filter-feeding Scyphomedusa from the northeast Gulf of Mexico. Estuarian, Coastal and Shelf Science 32: 511–525
Larson RJ and Arneson AC (1990) Two medusae new to the coast of California: Carybdea marsupialis (Linnaeus, 1758), a cubomedusa and Phyllorhiza punctata von Lendenfeld, 1884, a Rhizostome scyphomedusa. Bulletin of the Southern California Academy of Sciences 89(3): 130–136
Lucas C Hand Lawes S (1998) Sexual reproduction of the scyphomedusa Aurelia aurita in relation to temperature and variable food supply. Marine Biology 131: 629–638
Mayer AG (1910) Medusae of the World. Vol III. The Scyphomedusae, pp 499–735. Carnegie Institution, Washington, DC
Mianzan HW and Cornelius PFS (1999) Cubomedusae and Scyphomedusae. In: Boltovskoy D(ed) South Atlantic Zooplankton, pp 513–559. Backhuys Publishers, Leiden, The Netherlands
Moreira MGBS (1961) Sobre Mastigias scintillae sp. Nov. (Scyphomedusae, Rhizostomeae) das costas do Brasil. Bolm Inst Oceanography 11: 5–30
Olesen NJ, Frandsen K and Riisgård HU (1994) Population dynamics, growth and energetics of jellyfish Aurelia aurita in a shallow fjord. Marine Ecology Progress Series 105: 9–18
Purcell JE (1985) Predation on fish eggs and larvae by pelagic cnidarians and ctenophores. Bulletin of Marine Science 37(2): 739–755
Purcell JE (1989) Predation on fish larvae and eggs by the hydromdeusa Aequorea victoria at a herring spawning ground in British Columbia. Canadian Journal of Fisheries and Aquatic Science 46: 1415–1427
Purcell JE (1997) Pelagic cnidarians and ctenophores as predators: selective predation, feeding rates, and effects on prey populations. Annals of the Institute of Oceanography 73(2): 125–137
Purcell JE and Arai MN (2001) Interactions of pelagic cnidarians and ctenophores with fish: a review. Hydrobiologia 451: 27–44
Purcell JE, Cresswell FP, Cargo DG and Kennedy VS (1991) Differential ingestion and digestion of bivalve larvae by the scyphozoan Chrysaora quinquecirrha and the ctenophore Mnemiopsis leidyi. Biological Bulletin 180: 103–111
Purcell JE, Nemazie DA, Dorsey SE, Houde ED and Gamble JC (1994) Predation mortality of bay anchovy Anchoa mitchilli eggs and larvae due to scyphomedusae and ctenophores in Chesapeake Bay. Marine Ecology Progress Series 114: 47–58
Purcell JE, Shiganova TA, Decker MB and Houde ED (2001) The ctenophore Mnemiopsis in native and exotic habitats: USestuaries versus the Black Sea basin. Hydrobiologia 451: 145–176
Rajagopal S, Nair NVK and Azariah J (1989) Some observations on the problem of jelly fish ingress in a power station cooling system at Kalpakkam, east coast of India. Mahasagar 22(4): 151–158
Rippingale RJ and Kelly SJ (1995) Reproduction and survival of Phyllorhiza punctata (Cnidaria: Rhizostomeae) in a seasonally fluctuating salinity regime in western Australian. Marine and Freshwater Research 46: 1145–1151
Rouse GW and Pitt K (2000) Ultrastructure of the sperm of Catostylus mosaicus and Phyllorhiza punctata (Scyphozoa, Cnidaria): Implications for sperm terminology and the inference of reproductive mechanisms. Invertebrate Reproduction and Development 38: 23–34
Ruiz GM, Carlton JT, Grosholz ED and Hines AH (1997) Global invasions of marine and estuarine habitats by non-indigenous species: mechanisms, extent, and consequences. American Zoologist 37: 621–632
Shanks AL and Graham WM (1988) Chemical defense in a scyphomedusa. Marine Ecology Progress Series 45: 81–86
Shiganova TA (1998) Invasion of the Black Sea by the ctenophore Mnemiopsis leidyi and recent changes in pelagic community structure. Fisheries Oceanography 7(3/4): 305–310
Shigonova TA and Bulgakova YV (2000) Effects of gelatinous plankton on Black Sea and Sea of Azov fish and their food resources. ICES Journal of Marine Science 57: 641–648
Strom SL and Strom MW (1996) Microplankton growth, grazing, and community structure in the northern Gulf of Mexico. Marine Ecology Progress Series 130: 229–240
Stoecker DK and Govoni JJ (1984) Food selection by young larval gulf menhaden (Brevoortia patronus) Marine Biology 80: 299–306
Suchman CL and Sullivan BK (1988) Vulnerability of the copepod Acartia tonsa to predation by the scyphomedusa Chrysaora quinquecirrha: effect of prey size and behavior. Marine Biology 132(2): 237–245
von Lendenfeld R (1884) Über eine Übergangsform zwischen Semostomen und Rhizostomen. Zoologischer Anzeiger 5: 380–383
Weber M, Townsend RT and Bierce R (1992) Environmental quality in the gulf of mexico: a citizen's guide. Center for Marine Conservation, Washington, DC, 132 pp
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Graham, W.M., Martin, D.L., Felder, D.L. et al. Ecological and economic implications of a tropical jellyfish invader in the Gulf of Mexico. Biological Invasions 5, 53–69 (2003). https://doi.org/10.1023/A:1024046707234
Issue Date:
DOI: https://doi.org/10.1023/A:1024046707234