Abstract
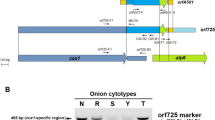
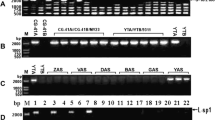
Cytoplasmic male sterile (CMS) plants were obtained by asymmetrical protoplast fusion between red cabbage (fertile) and normal radish (fertile). The CMS plants showed restriction fragment length polymorphism (RFLP) patterns of mitochondrial (mt) DNA that were different from those of both parental lines. PCR analysis of mtDNA of the CMS plants and though Southern blot analysis showed that the mtDNA of the CMS line had the characteristics of the ogura CMS type. The results suggested that the orf138 gene and the ogura type atp6 gene are present in normal fertile cabbage and radish at a low copy level.
Similar content being viewed by others
References
Aldrich, J. & C.A. Cullis, 1993. RAPD analysis in flax: Optimization of yield and reproducibility using KlenTaq 1 DNA polymerase, Chelex 100, and gel purification of genomic DNA. Plant Mol Biol Rep 11: 128-141.
Bonen, L., S. Bird & L. Belanger, 1990. Characterization of the wheat mitochondrial orf25 gene. Plant Mol Biol 15: 793-795.
Estiati, A., T. Kubo & T. Mikami, 1998. The ribosomal S7 gene is transcribed and edited in sugar beet mitochondria. Phys Plant 102: 325-327.
Fu, T.D., 1981. Production and research of rapeseed in the People's Republic of China. Eucarpia Cruciferae Newsletter 6: 6-7.
Gualbert, J.M., C. Domon, J. Weil & J. Grienenberger, 1990. Structure and transcription of the gene coding for subunit 3 of cytochrome oxidase in wheat Mitochondria. Curr Genet 17: 41-47.
Hartmann, C., Y. Henry, J. De Buyser, C. Aubry & A. Rode, 1989. Identification of new mitochondrial genome organizations in wheat plants regenerated from somatic tissue cultures. Theor Appl Genet 77: 169-175.
Kameya, T., H. Kanzaki, S. Toki & T. Abe, 1989. Transfer of radish (Raphanus sativus L.) chloroplasts into cabbage (Brassica oleracea L.) by protoplast fusion. Jpn J Genet 64: 27-34.
Kanazawa, A., N. Tsutsumi & A. Hirai, 1994. Reversible changes in the composition of the population of mtDNAs during dedifferentiation and regeneration in tobacco. Genetics 138: 865-870.
Kanno, A., H. Kanzaki & T. Kameya, 1997. Detailed analysis of chloroplast and mitochondrial DNAs from the hybrid plant generated by asymmetric protoplast fusion between radish and cabbage. Plant Cell Rep 16: 479-484.
Krishnasamy, S. & C.A. Makaroff, 1994. Organ-specific reduction in the abundance of a mithochondrial protein accompanies fertility restoration in cytoplasmic male-sterile radish. Plant Mol Biol 26: 935-946.
Kubo, T., T. Mikami & T. Kinoshita, 1993. The sugar beet mitochondrial genome contains an ORF sharing sequence homology with the gene for the 30kDa subunit of bovine mitochondrial complex I. Mol Gen Genet 241: 479-482.
Kubo, N., K. Ozawa, T. Hino & K. Kadowaki, 1996. A ribosomal protein L2 gene is transcribed, spliced and edited at one site in rice mitochondria. Plant Mol Biol 31: 853-862.
Ogura, H., 1968. Studies on the new male sterility in Japanese radish, with special references to the utilization of this sterility towards practical raising of hybrid seed. Mem Fac Agric Kagoshima Univ 6: 39-78.
Ozias-Akins, P., Z. Tabaeizadeh, D.R. Pring & I.K. Vasil, 1998. Preferential amplification of mitochondrial DNA fragments in somatic hybrids of the Gramineae. Curr Genet 13: 241-245.
Rose, R.J., L.B. Johnson & R.J. Kemble, 1986. Restriction endonuclease studies on the chloroplast and mitochondrial DNAs of alfalfa (Medicago sativa L.) protoclones. Plant Mol Biol 6: 331-338.
Sakai, T. & J. Imamura, 1993. Evidence for a mitochondrial subgenome containing radish atpA in a Brassica napus cybrid. Plant Sci 90: 95-103.
Shiga, T., 1980. Male sterility and cytoplasmic differentiation. In: Brassica Crops and Wild Allies-Biology and breeding, Chap. 12, pp. 205-221. Japan Sci Soc Press, Tokyo.
Shirzadegan, M., J.D. Palmer, M. Christey & E.D. Earle, 1991. Patterns of mitochondrial DNA in Brassica campestris cultured cells. Plant Mol Biol 16: 21-37.
Souza, A.P. de, M. Jubier, E. Delcher, D. Lancelin & B. Lejeune, 1990. A trans-splicing model for the expression of the tripartite nad5 gene in wheat and maze mitochondria. Plant Cell 3: 1363-1378.
Small, I.D., R. Suffolk & C.J. Leaver, 1989. Evolution of plant mitochondrial genomes via substoichiometric intermediates. Cell 58: 69-76.
Suzuki, T., S. Kazama, A. Hirai, T. Akihama & K. Kadowaki, 1991. The rice mitochondrial nap3 gene has an extended reading frame at its 5' end: nucleotide sequence analysis of rice trnS, nad3, and rps12 genes. Curr Genet 20: 331-337.
Tai Y. Huh & M.W. Gray, 1982. Conservation of ribosomal RNA gene arrangement in the mitochondrial DNA of angiosperms. Plant Mol Biol 1: 245-249.
Thompson, K.F., 1972. Cytoplasmic male sterility in oilseed rape. Heredity 29: 253-257.
Umbeck, P.F. & B.G. Gengenbach, 1983. Reversion of male-sterile T-cytoplasm maize to male fertility in tissue culture. Crop Sci 23: 384-388.
Yamagishi, H. & T. Terachi, 1996. Molecular and biological studies on male sterile cytoplasm in the Cruciferae. III. Distribution of Ogura-cytoplasm among Japanese wild radishes and Asian radish cultivars. Theor Appl Genet 93: 325-332.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Motegi, T., Sup Nou, I., Zhou, J. et al. Obtaining an Ogura-type CMS line from asymmetrical protoplast fusion between cabbage (fertile) and radish (fertile). Euphytica 129, 319–323 (2003). https://doi.org/10.1023/A:1022284803689
Issue Date:
DOI: https://doi.org/10.1023/A:1022284803689