Abstract
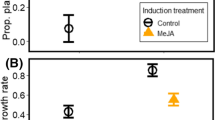
The role of induced responses of tomato, Lycopersicon esculentum, in interspecific interactions between two polyphagous herbivores, the silverleaf whitefly, Bemisia argentifolii (WF), and the vegetable leafminer, Liriomyza trifolii (LM), was characterized in laboratory and field experiments. Feeding by LMs and WFs induced local and systemic production of putative defensive proteins, i.e., chitinases, peroxidases, β-1,3-glucanases, and lysozymes. The magnitude of the induction for each defensive protein varied between species. Unlike WFs, LMs caused a 33% local reduction in total foliar protein content. In a whole-plant choice experiment, adult LM feeding, oviposition, and larval survival were reduced by 47.7%, 30.7%, and 26.5%, respectively, for the WF-infested host compared with the controls. Early WF infestations also had negative systemic (plant-mediated) effects on LMs. Adult LMs preferred leaves from control plants to leaves of plants that had been previously infested with WFs; no reciprocal effect of LMs on WFs were found. Feeding by Helicoverpa zea larvae, which has been shown previously to affect LM performance, had no effect on WF survival and development. LM natural population dynamics were monitored on WF-preinfested and control plants in a field experiment. WF-infested plants were less suitable for LM development with an overall 41% reduction in LM population density. These results demonstrate asymmetric direct and plant-mediated interspecific interactions between generalist herbivores feeding simultaneously on the same host. Possible mechanisms by which WFs overcome plant defenses are suggested. This ability may also contribute to WF success that makes them a major pest worldwide. The study supports the idea that over an evolutionary time scale, herbivores sharing the same host plant will automatically compete.
Similar content being viewed by others
REFERENCES
Agrawal, A. A. 1998. Induced response to herbivory and increased plant performance. Science 279:1201-1202.
Alborn, H. T., Turlings, T. C. J., Jones, T. H., Stenhagen, G., Loughrin, J. H., and Tumlinson, J. H. 1997. An elicitor of plant volatiles from beet armyworm oral secretion. Science 276:945-949.
Baldwin, I. T. 1994. Chemical changes rapidly induced by folivory, pp. 2-16, in E. A. Bernays, (ed.). Insect Plant Interactions, Vol. 5. CRC Press, Boca Raton, Florida.
Berenbaum, M. R. 1991. Coumarins, pp. 221-249, in G. A. Rosenthal and M. R. Berenbaum (eds.). Herbivores: Their Interactions with Secondary Plant Metabolites, 2nd ed. Academic Press, San Diego.
Bowles, D. J. 1990. Defense-related proteins in higher plants. Annu. Rev. Biochem. 59:873-907.
Byrne, D. N., and Bellows, T. S., Jr. 1991. Whitefly biology. Annu. Rev. Entomol. 36:431-457.
Carozzi, N., and Koziel, M. 1997. Advances in Insect Control: The Role of Transgenic Plants. Taylor & Francis, London.
Cohen, A. C., Henneberry, T. J., and Chu, C. C. 1996. Geometric relationships between whitefly feeding behavior and vascular bundle arrangements. Entomol. Exp. Appl. 78:135-142.
Danell, K., Huss-Danell, K., and Bergstrom, R. 1985. Interactions between browsing moose and two species of birch in Sweden. Ecology 66:1687-1878.
Denno, R. F., McClure, M. S., and Ott, J. R. 1995. Interspecific interactions in phytophagous insects: Competition reexamined and resurrected. Annu. Rev. Entomol. 40:297-331.
Duffey, S. S., and Felton, G. W. 1991. Enzymatic antinutritive defenses of the tomato plant against insects, pp. 166-197, in P. A. Hedin (ed.). Naturally Occurring Pest Bioregulators. ACS Symposium Series 449, Dallas, Fall 1989. American Chemical Society, Washington, D.C.
Duffey, S. S., and Stout, M. J. 1996. Antinutritive and toxic components of plant defense against insects. Arch. Insect Biochem. Physiol. 32:3-37.
Edwards, P. J., Wratten, S. D., and Cox, H. 1985. Wound induced changes in the acceptability of tomato to larvae of Spodoptera littoralis: A laboratory bioassay. Econ. Entomol. 10:155-158.
Faeth, S. H. 1986. Indirect interactions between temporally separated herbivores mediated by the host plant. Ecology 67:479-494.
Felton, G. W., Summers, C. B., and Mueller, A. J. 1994. Oxidative responses in soybean foliage to herbivory by bean leaf beetle and three-cornered alfalfa hopper. J. Chem. Ecol. 20:639-649.
Fowler, S. V., and Lawton, J. H. 1985. Rapidly induced defenses and talking trees: The devil's advocate position. Am. Nat. 126:181-195.
Gerling, D., and Mayer, R. T. 1996. Bemisia 1995: Taxonomy, Biology, Damage Control and Management. Intercept Ltd., Andover, Hants., UK.
Harrison, S., and Karban, R. 1986. Effects of an early-season folivorous moth on the success of a later-season species, mediated by a change in the quality of the shared host, Lupinus arboreus Sims. Oecologia 69:354-359.
Hartley, S. E., and Lawton, J. H. 1991. Biochemical aspects and significance of the rapidly induced accumulation of phenolics in birch foliage, pp. 105-132, in D. W. Tallamy and M. J. Raupp (eds.). Phytochemical Induction by Herbivores, John Wiley & Sons, New York.
Haukioja, E. 1990. Induction of defenses in trees. Annu. Rev. Entomol. 36:25-42.
Hougen-Eitzman, D., and Karban, R. 1995. Mechanisms of interspecific competition that result in successful control of Pacific mites following inoculations of Willamette mites on grapevines. Oecologia 103:159-161.
Hunter, M. D. 1992. Interactions within herbivores communities mediated by the host plant: the keystone herbivore concept, pp. 287-325, in M. D. Hunter, P. W. Price, and T. Ohgushi (eds.). Effects of Resource Distribution on Animal-Plant Interactions. Academic Press, San Diego.
Inbar, M., Eshel, A., and Wool, D. 1995. Interspecific competition among phloem-feeding insects mediated by induced host-plant sinks. Ecology 76:1506-1515.
Inbar, M., Doostdar, H. R., Sonoda, M., Leibee, G. L., and Mayer, R. T. 1998. Elicitors of plant defensive systems reduce insect densities and disease incidence. J. Chem. Ecol. 24:135-149.
Inbar, M., Doostdar, H., and Mayer, R. T. 1999. The effects of sessile insects (whitefly nymphs) on leaf-chewing caterpillars. Environ. Entomol. 28(3):353-357.
Janzen, D. H. 1973. Host plants as islands. II. Competition in evolutionary and contemporary time. Am. Nat. 107:786-790.
Jones, C. G., Hopper, R. F., Coleman, J. S., and Krischik, V. A. 1993. Control of systemically induced herbivore resistance by plant vascular architecture. Oecologia 93:452-456.
Karban, R., and Baldwin, I. T. 1997. Induced Response to Herbivory. University of Chicago Press, Chicago.
Karban, R., and Myers, J. H. 1989. Induced plant responses to herbivory. Annu. Rev. Ecol. Syst. 20:331-348.
Karban, R., Adamchak, R., and Schnathorst, W. C. 1987. Induced resistance and interspecific competition between spider mites and a vascular wilt fungus. Science 235:678-680.
Kogan, M., and Fischer, D. C. 1991. Inducible defenses in soybean against herbivorous insects, pp. 347-378, in D. W. Tallamy and M. J. Raupp (eds.). Phytochemical Induction by Herbivores. John Wiley & Sons, New York.
Little, T. M., and Hills, J. F. 1978. Agricultural Experimentation: Design and Analysis. John Wiley & Sons, New York.
Liu, T.-X., and Stansly, P. A. 1995. Oviposition by Bemisia argentifolii (Homoptera: Aleyrodidae) on tomato: Effects of leaf factors and insecticide residue. J. Econ. Entomol. 88:992-997.
Martinsen, G. D., Driebe, E. M., and Whitham, T. G. 1998. Indirect interactions mediated by changing plant chemistry: Beaver browsing benefits beetles. Ecology 79:192-200.
Masters, G. J., Brown, V. K., and Gange, A. C. 1993. Plant mediated interactions between above-and below-ground insect herbivores. Oikos 66:148-151.
Mayer, R. T., McCollum, T. G., McDonald, R. E., Polston, J. E., and Doostdar, H. 1996. Bemisia feeding induces pathogenesis-related proteins in tomato, pp. 179-188, in D. Gerling and R. T. Mayer (eds.). Bemisia 1995: Taxonomy, Biology, Damage Control and Management. Intercept Ltd., Andover, Hants., UK.
McKey, D. 1979. The distribution of secondary compounds within plants, pp. 55-133, in G. A. Rosenthal and D. H. Janzen (eds.). Herbivores, Their Interaction with Secondary Plant Metabolites. Academic Press, New York.
Minkenberg, O. P. J. M., and Ottenheim, J. J. G. W. 1990. Effect of leaf nitrogen content of tomato plants on preference and performance of a leafmining fly. Oecologia 83:291-298.
Mullin, C. A. 1986. Adaptive divergence of chewing and sucking arthropods to plant allelochemicals, pp. 175-209, in L. B. Brattsten and S. Ahmad (eds.). Molecular Aspects of Insect-plant Associations. Plenum Press, New York.
Parrella, M. P. 1987. Biology of Liriomyza. Annu. Rev. Entomol. 32:201-224.
Price, P. W., Bouton, C. E., Gross, P., McPheron, B. A., Thompson, J. N., and Weis, A. E. 1980. Interactions among three trophic levels: Influence of plants on interactions between insect herbivores and natural enemies. Annu. Rev. Ecol. Syst. 11:41-65.
Rosenheim, J. A., Johnson, M. W., Mau, R. F. L., Welter, S. C., and Tabashnik, B. E. 1996. Biochemical preadaptations, founder events, and the evolution of resistance in arthropods. J. Econ. Entomol. 89:263-273.
SAS Institute. 1988. SAS/STAT User's Guide. Release 6.11. SAS Institute, Cary, North Carolina.
Schuster, D. J. 1998. Intraplant distribution of immature lifestages of Bemisia argentifolii (Homoptera: Aleyrodidae) on tomato. Environ. Entomol. 27:1-9.
Schuster, D. J., and Beck, H. W. 1992. Presence-absence sampling for assessing densities of larval leafminers in field-grown tomatoes. Trop. Pest Manage. 38:254-256.
Stout, M. J., and Duffey, S. S. 1996. Characterization of induced resistance in tomato plants. Entomol. Exp. Appl. 79:273-283.
Stout, M. J., Workman, K. V., and Duffey, S. S. 1994. Differential induction of tomato foliar proteins by arthropod herbivores. J. Chem. Ecol. 20:2575-2594.
Stout, M. J., Workman, K. V., and Duffey, S. S. 1996. Identity, spatial distribution, and variability of induced chemical responses in tomato plants. Entomol. Exp. Appl. 79:255-271.
Strauss, S. Y. 1991. Indirect effects in community ecology: Their definition, study, and importance. Trends Ecol. Evol. 6:206-210.
Strong, D. R., Lawton, J. H., and Southwood, R. 1984. Insect on plants: Community patterns and mechanisms. Harvard University Press, Cambridge, Massachusetts.
Tallamy, D. W., and Raupp, M. J. 1991. Phytochemical Induction by Herbivores. John Wiley & Sons, New York.
van Loon, L. C., Piepoint, W. C., Boller, T., and Conejero, V. 1994. Recommendations for naming plant pathogenesis-related proteins. Plant. Mol. Biol. Rep. 12:245-264.
Walker-Simmons, M., and Ryan, C. A. 1977. Immunological identification of proteinase inhibitors I and II in isolated tomato leaf vacuoles. Plant Physiol. 60:61-63.
Wang, X., Ding, X., Gopalakrishnan, B., Morgan, T. D., Johnson, L., White, F. F., Muthukrishnan, S., and Kramer, K. J. 1996. Characterization of a 46 kDa insect chitinase from transgenic tobacco. Insect Biochem. Mol. Biol. 26:1055-1064.
Wilkens, R. T., Spoerke, J. M., and Stamp, N. E. 1996. Differential responses of growth and two soluble phenolics of tomato to resource availability. Ecology 77:247-258.
Wootton, T. J. 1994. The nature and consequences of indirect effects in ecological communities. Annu. Rev. Ecol. Syst. 25:443-466.
Zangerl, A. R. 1990. Furanocoumarin induction in wild parsnip: Evidence for an induced defense against herbivores. Ecology 71:1926-1932.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Inbar, M., Doostdar, H., Leibee, G.L. et al. The Role of Plant Rapidly Induced Responses in Asymmetric Interspecific Interactions Among Insect Herbivores. J Chem Ecol 25, 1961–1979 (1999). https://doi.org/10.1023/A:1020998219928
Issue Date:
DOI: https://doi.org/10.1023/A:1020998219928