Abstract
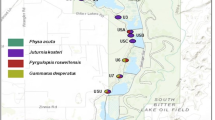
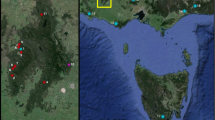
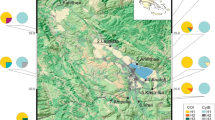
During the Pleistocene, when the climate was wetter and cooler, aquatic habitats in the Great Basin of western North America were much more extensive and connected. As the climate warmed over the last 10 000 years, many of these habitats dried but others remained as isolated springs and inland lakes. The isolation of desert springs and lack of dispersal between populations of non-vagile species (e.g. fish, spring snails) has led to genetic differentiation and speciation. However, the extent to which vagile species of aquatic insects disperse from spring to spring is unknown. We examined the population genetics of two caddisflies, Hesperophylax designatus (Limnephilidae) and Lepidostoma ojanum (Lepidostomatidae) that occur in isolated springs in Nevada and eastern California to determine the extent of their dispersal from spring to spring. Mitochondrial DNA sequences indicate that the populations of L. ojanum are isolated and that the populations represent management units. In contrast, H. designatus individuals are flying from spring to spring and their populations are connected by dispersal. Disturbance impacts (e.g. grazing by ungulates, water extraction) that eliminate poor dispersers (e.g. L. ojanum) locally may result in permanent losses of genetic diversity; this is less likely with broader dispersers such as H. designatus.
Similar content being viewed by others
References
Anderson, N.H. (1976) The distribution and biology of Oregon Trichoptera. Oregon Agric. Exp. Station Tech. Bull. 134, 1-160.
Clary, D.O. and Wolstenholme, D.R. (1985) The mitochondrial DNA molecule of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J. Mol. Evol. 22, 252-71.
Colburn, E.A. (1984) Diapause in a salt-tolerant desert caddisfly: the life cycle of Limnephilus assimilis (Trichoptera) in Death Valley. Am. Midland Naturalist 111, 280-87.
Cole, G.A. and Watkins, R.L. (1977) Hyallela montezuma, a new species (Crustacea: amphipoda) from Montezuma Well, Arizona. Hydrobiologia 52, 175-84.
Crichton, M.I. (1971) A study of caddis flies (Trichoptera) of the family Limnephilidae, based on the Rothamsted Insect Survery, 1964-68. J. Zool., London 163, 533-63.
Erman, N.A. and Erman, D.C. (1990) Biogeography of caddisfly (Trichoptera) assemblages in cold springs of the Sierra Nevada (California, USA). California Water Resources Center Contribution 200.
Erman, N.A. and Erman, D.C. (1995) Spring permanence, Trichoptera species richness, and the role of drought. J. Kansas Entomol. Soc. (Suppl.) 68, 50-64.
Excoffier, L., Smouse, P. and Quattro, J. (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131, 479-91.
Ferrington, L.C. (ed) (1995) Biodiversity of aquatic insects and other invertebrates in springs. J. Kansas Entomol. Soc. 68, 1-165.
Griffith, M.B., Barrows, E.M. and Perry, S.A. (1998) Lateral dispersal of adult aquatic insects (Plecoptera, Trichoptera) following emergence from headwater streams in forested Appalachian catchments. Entomol. Soc. Am 91, 195-201.
Hansen, T.A. (1980) Influence of larval dispersal and geographic distribution on species longevity in neogastropods. Paleobiology 6, 193-207.
Hershler, R. (1989) Springsnails (Gastropoda: Hydrobiidae) of Owens and Amargosa River (Exclusive of Ash Meadows) drainages, Death Valley System, California-Nevada. Proc. Biol. Soc. Washington 102, 176-248.
Hershler, R. (1998) A systematic review of the hydrobiid snails (Gastropoda: Rissooidea) of the Great Basin, Western United States, Part I. Genus Pyrgulopsis. Veliger 41, 1-132.
Hogg, I.D., Eadie, J.M. and De Lafontaine, Y. (1998) Atmospheric change and the diversity of aquatic invertebrates: are we missing the boat? Environ. Monitor. Assessment 49, 291-301.
Jackson, J.K. and Resh, V.H. (1989) Distribution and abundance of adult aquatic insects in the forest adjacent to a Northern California stream. Environ. Entomol. 18, 278-83.
Kovats, Z.E., Ciborowski, J.J.H. and Corkum. L.D. (1996) Inland dispersal of adult aquatic insects. Freshwater Biol. 36, 265-76.
La Rivers, I. (1953) New gelastocorid and naucorid records and miscellaneous notes, with a description of the new species, Ambrysus amargosus (Hemiptera: Naucoridae). Wassmann J. Biol. 11, 83-96
Lefkovitch, L.P. and Fahrig, L. (1985) Spatial characteristics of habitat patches and population survival. Ecol. Model. 30, 297-308.
Moritz, C. (1994) Defining ‘Evolutionarily Significant Units’ for conservation. Trends in Ecol. and Evol. 9, 373-75.
Myers, M.J. (2000) Aquatic invertebrates in island habitats: dispersal, population genetics, and ecology. Ph.D. dissertation, University of California, Berkeley, California.
Myers, M.J. and Resh, V.H. (1999a) Use of pan traps to collect adult Trichoptera in high desert spring habitats of California, USA. In Proceedings of the 9th International Symposium on Trichoptera. (H. Malicky and P. Chantaramongkol, eds), pp. 259-67. Faculty of Science, Chiang Mai University, Chiang Mai, Thailand.
Myers, M.J. and Resh, V.H. (2000) Undercut banks: a habitat for more than just fish. Trans. Am. Fisheries Soc. 129, 594-97.
Myers, M.J. and Resh, V.H. (2002) Trichoptera and other macroinvertebrates in springs of the Great Basin: species composition, richness, and distribution.Western North Am Naturalist (in press).
Myers, M.J., Meyer, C.P. and Resh, V.H. (2000) Neritid and thiarid gastropods from French Polynesian streams: how reproduction (sexual, parthenogenetic) and dispersal (active, passive) affect population structure. Freshwater Biol. 43, 1-11.
Parker, C.R. and Wiggins, G.B. (1985) The Nearctic genus Hesperophylax Banks (Trichoptera: Limnephilidae). Can. J. Zool. 63, 2443-72.
Polhemus, J.T. and Polhemus, D.A. (1994) A new species of Ambrysus Stål from Ash Meadows, Nevada (Heteroptera: Naucoridae). J. N.Y. Entomol. Soc. 102, 261-65
Ross, H.H. (1944) The caddis flies, or Trichoptera, of Illinois. Bull. Illinois Nat. History Surv. 23, 1-326.
Sada, D.W., Britten, H.B. and Brussard, P.F. (1995) Desert aquatic ecosystems and the genetic and morphological diversity of Death Valley system Speckled Dace. Am. Fisheries Soc. Symp. 17, 350-59
Schneider, S., Kueffer, J-M, Roessli, D. and Excoffier, L. (1997) Arlequin ver 1.1: A software for population genetic data analysis. Genetics and Biometry Laboratory, University of Geneva, Switzerland.
Shepard, W.D. (1990) Microcylloepus formicoideus (Coleoptera: Elmidae), a new riffle beetle from Death Valley National Monument, California. Entomol. News 101, 147-53.
Shepard, W.D. (1992) Riffle beetles (Coleoptera: Elmidae) of Death Valley National Monument, California. Great Basin Naturalist 52, 378-81.
Shepard, W.D. (1993) Desert springs-both rare and endangered. Aquatic Conservation: Mar. and Freshwater Ecosystems 3, 351-59.
Simon, C., Frati, F., Beckenbach, A., Crespi, B., Liu, H. and Flook, P. (1994) Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 87, 651-701.
Sode, A. and Wiberg-Larsen, P. (1993) Dispersal of adult Trichoptera at a Danish forest brook. Freshwater Biol. 30, 439-46.
Sokal, R.R. and Rohlf, F.J. (1995) Biometry.W.H. Freeman and Company, New York.
Sperling, F.A.H., Raske, A.G. and Otvos, I.S. (1999) Mitochondrial DNA sequence variation among populations and host races of Lambdina fiscellaria (Gn.) (Lepidoptera: Geometridae). Insect Mol. Biol. 8, 97-106.
Svensson, B.W. (1974) Population movements of adult Trichoptera at a south Swedish stream. Oikos 25, 157-75.
Swofford, D.L. (1999) PAUP * : Phylogenetic Analysis Using Parsimony ver4.0b2a. Sinauer, Sunderland, Massachusetts.
Thomas, E.P., Blinn, D.W. and Keim, P. (1997) Genetic and behavioural divergence among spring amphipod populations. Freshwater Biol. 38, 137-43.
Weaver, J.S. III and Myers, M.J. (1998) Two new species of caddisflies of the genus Lepidostoma Rambur (Trichoptera: Lepidostomatidae) from the Great Basin. Aquatic Insects 20, 189-95.
Wiggins, G.B. (1996) Larvae of the North American Caddisfly Genera (Trichoptera). University of Toronto Press, Toronto, Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Myers, M.J., Sperling, F. & Resh, V. Dispersal of Two Species of Trichoptera from Desert Springs: Conservation Implications for Isolated vs Connected Populations. Journal of Insect Conservation 5, 207–215 (2001). https://doi.org/10.1023/A:1017998513721
Issue Date:
DOI: https://doi.org/10.1023/A:1017998513721