Abstract
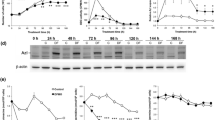
Data derived from models of hepatic regeneration indicate that transient, recipricol changes in polyamines, potent growth promoters, and gamma aminobutyric acid (GABA), an amino acid neurotransmitter with growth inhibitory properties, play important roles in enhancing and inhibiting respectively regulated hepatocyte proliferation. Based on these findings and supportive data derived from studies of human carcinoma tissues and malignant cell lines we propose that permanent increases in polyamine and decreases in GABAergic activity act in concert to contribute to the pathogenesis of hepatocellular carcinoma.
Similar content being viewed by others
References
London WT:Primary hepatocellular carcinoma-etiology, pathogenesis, and prevention. Hum Pathol 12: 1085–1097, 1981
Di Bisceglie AM, Rustgi VK, Hoofnagle JH, Dusheiko GM, Lotze MT: Hepatocellular carcinoma. Ann Intern Med 108: 390–401, 1988
Hobbs KEF, Dusheiko GM: Management of Hepatocellular carcinoma. J Hepatol 15: 281–283, 1992
Okuda K: Hepatocellular carcinoma: Recent progress. Hepatology 15: 948–963, 1992
Pegg AE, McCann PP: Polyamine metabolism and function. Am J Physiol 243: C212-C221, 1982
Luk GD: Essential role of polyamine metabolism in hepatic regeneration: inhibition of deoxyribonucleic acid and protein synthesis and tissue regeneration by difluoromethylornithine in the rat. Gastroenterology 90: 1261–1267, 1986
Russell DH, Snyder SH: Amine synthesis in regenerating rat liver: extremely rapid turnover of ornithine decarboxylase. Mol Pharmacol 5: 253–262, 1969
Minuk GY, Gauthier T, Benarroch A: Changes in serum and hepatic polyamine concentrations following 30%, 70% and 90% partial hepatectomy in rats. Hepatology 12: 542–546, 1990
Nishiguchi S, Kuroki T, Takeda T, Nakajima S, Shiomi S, Seki S, Matsui-Yuasa I, Otani S, Kobayashi K: Effects of putrescine on D-galactosamine-induced acute liver failure in rats. Hepatology 12: 348–353, 1990
Diehl AM, Abdo S, Braun N: Supplemental putrescine reverses ethanol-associated inhibition of liver regeneration. Hepatology 12: 633–637, 1990
Janne J, Alhonen L, Leinonen P: Polyamines: from molecular biology to clinical applications. Ann Med 23: 241–259, 1991
Haddox MK, Russell DH: Increased nuclear conjugated polyamines and transglutaminase during liver regeneration. Proc Natl Acad Sci USA 78: 1712–1716, 1981
Poso JH, Raina A: Polyamines in rapid growth and cancer. Biochim Biophys Acta 473: 241–293, 1978
Minuk GY, Kren BT, Xu R, Zhang XK, Burczynski FJ, Mulrooney NP, Fan G, Steer CJ: The effect of changes in hepatocyte membrne potential on immediate-early protooncogene expression following partial hepatectomy in rats. Hepatology 25: 1123–1127, 1997
Zhang XK, Gauthier T, Burczynski FJ, Wang GQ, Gong Y, Minuk GY: Changes in liver membrane potentials after partial hepatectomy in rats. Hepatology 23: 549–551, 1996
Minuk GY, Bear CE, Sarjeant EJ: Sodium independent bicuculline sensitive, [3H] GABA binding to isolated rat hepatocytes. Am J Physiol 252: 642–647, 1987
Stephenson FA: The GABAA receptors. Biochem J 310: 1–9, 1995
Nelson MT, Blaustein MP: GABA efflux from synaptosomes: effects of membrane potential and external GABA and cations. J Membrane Biol 69: 213–223, 1982
Herbison AE: Estrogen regulation of GABA transmission in rat preoptic area. Brain Research Bulletin 44(4) 321–326, 1997
Minuk GY: Gamma-aminobutyric acid and the liver Dig Dis 11: 45–54, 1993
Minuk GY, Gauthier T: The effect of gamma aminobutyric acid (GABA) on hepatic regenerative activity following partial hepatectomy in rats. Gastroenterology 104: 217–221, 1993
Zhang XK, Gauthier T, Burczynski FJ, Wang GQ, Gong Y, Minuk GY: Ciprofloxacin prevents the inhibitor effects of acute ethanol exposure on hepatic regeneration in the rat. Hepatology 22: 1797–1800, 1995
Kaita KDE, Assy N, Gauthier T, Meyers AFA, Minuk GY: Beneficial effects of ciprofloxacin on survival and hepatic regenerative activity in a rat model of fulminant hepatic failure. Hepatology 27: 533–536, 1998
Zhang M, Song G, Minuk GY: Effects of hepatic stimulator substance, herbal medicine, selenium/vitamin E, and ciprofloxacin on cirrhosis in the rat. Gastroenterology 110: 1150–1155, 1996
Janne J, Poso H, Raina A: Polyamines in rapid growth and cancer. Biochim Biophys Acta 473: 241–293, 1978
Auvinen M, Paasinen A, Andersson LC, et al: Ornithine decarboxylase activity is critical for cell transformation. Nature 360: 355–358, 1992
Don S, Bachrach U: Polyamine metabolism in normal and in virus-transformed chick embryo fibroblasts. Cancer Res 35: 3618–3622, 1975
Tamori A, Nishiguchi S, Kuroki T, Seki S, Kobayashi K, Kinoshita H, Otani S: Relationship of ornithine decarboxylase activity and histological findings in human hepatocellular carcinoma. Hepatology 20: 1179–1186, 1994
Sunkara PS, Baylin SB, Luk GD: Inhibitors of polyamine biosynthesis: Cellular and in vivo effects on tumor proliferation. In: P.P. McCann, A.E. Pegg, A. Sjoerdsma (eds). Inhibition of Polyamine Metabolism: Biological Significance and Basis for New Therapies. Academic Press, San Diego, 1987, pp 2–36
Manni A, Badger B, Lynch J, Demers L: Selectivity of polyamine involvement in hormone action on normal and neoplastic target tissues of the rat. Breast Cancer Res Treat 17: 187–196, 1990
Kim I, Manni A, Lynch J, Demers L: Polyamine involvement in the secretion and action of TGF-α in hormone sensitive human breast cancer cells in culture. Breast Cancer Res Treat 18: 83–91, 1991
Holtta E, Sistonen L, Alitalo K: The mechanisms of ornithine decarboxylase deregulation in c-Ha-ras oncogene-transformed NIH 3T3 cells. J Biol Chem 263: 4500–4507, 1988
Boggust WA, Al-Nakib T: Promotion and suppression of tumour growth and cell proliferation by acetylputrescine and putrescine and their oxidation products acetyl-GABA and GABA. IRCS Med Sci 14: 174–175, 1986
Cerino A, De Amici M, Fussi FF, Ricotti GCBA: Carboxyethyl γ-aminobutyric acid, a polyamine derivative molecule with a growth effect on hybridomas. J Immunol Methods 77: 229–235, 1985
Tsutsumi I, Ido A, Nakao K, Hamasaki K, Kato Y, Ohtsuru A, Nakata K, Tamaoki T, Nagataki S: Reciprocal regulation of afegoprotein and albumin gene expression by butyrate in human hepatoma cells. Gastroenterology 107: 499–504, 1994
Leder A, Leder P: Butyric acid, a potent inducer of erythroid differentiation in cultured erythroleukemic cells. Cell 5: 319–322, 1975
Langdon SP, Hawkes MM, Hay FG, Lawrie SS, Schiol DJ, Hilgers J, Leonard RCF, Smyth JF: Effect of sodium butyrate and other differentiation inducers on poorly differentiated human ovarian adenocarcinoma cell lines. Cancer Res 48: 6161–6165, 1988
McGovren JP, Williams MG, Tang AH, Vonvoigtland PF, Piercey MF, Einspahr FJ, Schreur PJ: Animal behavioral and neurochemical effects of the CNS toxic amino acid antitumor agent, acivicin. Res Commun Chem Pathol Pharmacol 63: 215–229, 1989
Marino AA, Iliev KG, Schwalke MA, Gonzalez E, Marler KC, Flanagan CA: Association between cell membrane potential and breast cancer. Tumor Biol 15: 82–89, 1994
Zhang M, Gong YW, Assy N, Minuk GY. Increased GABAergic activity inhibits α-fetoprotein mRNA expression and the proliferative activity of the Hep G2 human hepatocellular carcinoma cell line. J Hepatol 32: 85–91, 2000
Shiina S, Tagawa K, Unuma T, Fujino H, Uta Y, Niwa Y, Hata Y, Komatsu Y, Shiratori Y, Terano A, Sugimoto T: Percutaneous ethanol injection therapy of hepatocellular carcinoma: Analysis of 77 patients. AJR 155: 1221–1226, 1990
Gong Y, Cui L, Zhang MN, Burczynski FJ, Minuk GY: Decreased GABAergic activity in human hepatocellular carcinoma. Hepatology 26: 131A, 1997
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Minuk, G. Gaba and hepatocellular carcinoma. Mol Cell Biochem 207, 105–108 (2000). https://doi.org/10.1023/A:1007062802164
Issue Date:
DOI: https://doi.org/10.1023/A:1007062802164