Abstract
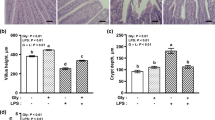
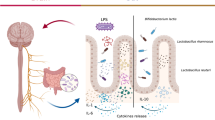
The hypothesis that lactoferrin protects mice against lethal effects of bacterial lipopolysaccharide (LPS) is the subject of experimental investigations described in this article. Lipopolysaccharide is a powerful toxin produced by Gram negative bacteria that when injected into humans or experimental animals reproduce many of the pathophysiologic and immune responses caused by live bacteria. Lactoferrin administered intraperitoneally 1 hr prior to injection of LPS significantly enhanced the survival of mice, reducing LPS-induced mortality from 83.3% to 16.7%. Changes in locomotor and other behavioral activities resulting from LPS injection were not present in mice treated with lactoferrin. Also, histological examination of intestine revealed remarkable resistance to injury produced by LPS if mice were pretreated with lactoferrin. Severe villus atrophy, edema and epithelial vacuolation were observed in LPS-treated animals but not in lactoferrin-treated counterparts. Electrophysiological parameters were used to assess secretory and absorptive functions in the small intestine. In mice treated with LPS, transmural electrical resistance was reduced and absorption of glucose was increased. Lactoferrin treatment had no significant influence on basal electrophysiological correlates of net ion secretion or glucose absorption nor on changes induced by LPS. Collectively, these results suggest that lactoferrin attenuates the lethal effect of LPS and modulates behavioral and histopathological sequela of endotoxemia.
Similar content being viewed by others
REFERENCES
REITER, B. 1983. The biological significance of lactoferrin. Intern. J. Tissue Reactions. 5:87-96.
SANCHEZ, L., M. CALVO, and J. H. BROCK. 1992. Biological role of lactoferrin. Arch. Dis. Child. 67:657-661.
LONNERDAL, B., and S. IYER. 1995. Lactoferrin: molecular structure and biological function. Annu. Rev. Nutr. 15:93-110.
BROCK, J. 1995. Lactoferrin: a multifunctional immunoregulatory protein? Immunol Today. 16:417-419.
BRITIGAN, B., J. S. SERODY, and M. S. COHEN. 1994. The role of lactoferrin as an anti-inflammatory molecule. In: Lactoferrin: Structure and Function. Ed. T. W. Hutchens et al., Plenum Press, New York. 143-155.
BULLEN, J. J. 1981. The significance of iron in infection. Rev. Infect. Dis. 3:1127-1138.
TOMITA, M., W. BELLAMY, M. TAKASE, K. YAMAUCHI, H. WAKABAYASHI, and K. KAWASE, 1991. Potent antibacterial peptides generated by pepsin digestion of bovine lactoferrin. J. Dairy Sci. 74:4137-4142.
BELLAMY, W., M. TAKASE, K. YAMAUCHI, H. WAKABAYASHI, K. KAWASE, and M. TOMITA. 1992. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta. 1121:130-136.
SAWATZKI, G., and I. N. RICH. 1989. Lactoferrin stimulates colony stimulating factor production in vitro and in vivo. Blood Cells 15:371-385.
ZIMECKI, M., and M. MACHNICKI. 1994. Lactoferrin inhibits the effector phase of delayed type hypersensitivity to sheep erythrocytes and inflammatory reactions to M. bovis (BCG). Arch. Immunol. Ther. Exp. 42:171-177.
ZIMECKI, M., J. MAZURIER, M. MACHNICKI, Z. WIECZOREK, J. MONTREUIL, and G. SPIK. 1991. Immunostimulatory activity of lactotransferrin and maturation of CD4-CD8-thymocytes. Immunol. Lett. 30:119-124.
ZIMECKI, M., J. MAZURIER, G. SPIK, and J. A. KAPP. 1995. Human lactoferrin induces phenotypic and functional changes in splenic mouse B cells. Immunology 86:112-127.
ZIMECKI, M., J. MAZURIER, G. SPIK and J. A. KAPP. 1996. Lactoferrin inhibits proliferative response and cytokine production by TH1 but not TH2 cells. Arch. Immunol. Ther. Exp. 44:51-56.
SORIMACHI, K., K. AKIMOTO, Y. HATTORI, T. IEIRI, and A. NIWA. 1997. Activation of macrophages by lactoferrin: secretion of TNFa, IL-8 and NO. Biochem. Mol. Biol. Internat. 43:79-87.
MIYAZAWA, K., C. MANTEL, L. LU, D. C. MORRISON, H. E. BROXMEYER. Lactoferrin-lipopolysaccharide interactions. Effect on lactoferrin binding to monocyte /macrophage-differentiated HL-60 cells. J. Immunol. 1991;146:723-729.
CROUCH, S. P. M., K. J. SLATER, and J. FLETCHER. 1992. Regulation of cytokine release from mononuclear cells by the iron-binding protein lactoferrin. Blood 80:235-240.
ELASS-ROCHARD, E., A. ROSEANU, D. LEGRAND, M. TRIF, V. SALMON, C. MOTAS, J. MONTREUIL, and G. SPIK. 1995. Lactoferrin lipopolysaccharide interaction: involvement of the 28-34 loop region of human lactoferrin in the high-affinity binding to Escherichia coli 055B5 lipopolysaccharide. Biochem. J. 312:839-845.
ELASS-ROCHARD, E., D. LEGRAND, V. SALMON, A. ROSEANU, M. TRIF, P. S. TOBIAS, J. MAZURIER, and G. SPIK. 1998. Lactoferrin inhibits the endotoxin interaction with CD14 by competition with the lipopolysaccharide-binding protein. Infect. Immun. 66:486-491.
COHEN, M. S., J. MAO, G. T. RASMUSSEN, J. S. SERODY, and B. BRITIGAN, 1992. Interaction of lactoferrin and lipopolysaccharide (LPS): Effects on the antioxidant property of lactoferrin and the ability of LPS to prime human neutrophils for enhanced superoxide formation. J. Infect. Dis. 166:1375-1378.
WANG, D., K. M. PABST, Y. AIDA and M. J. PABST. 1995. Lipopolysaccharide-inactivating activity of neutrophils is due to lactoferrin. J. Leuk. Biol. 57:865-874.
MACHNICKI, M., M. ZIMECKI and T. ZAGULSKI. 1993. Lactoferrin regulates the release of tumor necrosis factor alpha and interleukin 6 in vivo. Int. J. Exp. Path. 74:433-439.
ZAGULSKI, T., P. LIPINSKI, A. ZAGULSKA, S. BRONIEK, and Z. JARZABEK. 1989. Lactoferrin can protect mice against lethal dose of Escherichia coli in experimental infection in vivo. Br. J. Exp. Path. 70:697-704.
LEE, W. J., J. L. FARMER, M. HILTY, and Y. B. KIM. 1998. The protective effects of lactoferrin feeding against endotoxin lethal shock in germfree piglets. Infect. Immun. 66:1421-1426.
BULLICK, G. R., R. A. FRIZZELL, and G. A. CASTRO. 1998. Trichinella spiralis: Rapid, Immunologically influenced Reduction of intestinal, Sodium-coupled sugar transport in rat. Experimental Parasitology 57:104-109.
DEITCH, E. A., and R. BERG. 1987. Endotoxin promotes the translocation of bacteria from the gut. Arch. Surg. 122:185-190.
DEITCH, E. A., and R. BERG, 1987. Endotoxin but not malnutrition promotes bacterial translocation in the gut flora in burn mice. J. Trauma., 27:161-166.
MAINOUS, M. R., W. ERTEL, I. H. CHAUDRY, and E. A. DEITCH. 1995. The gut: a cytokinegenerating organ in systemic inflammation? Shock 4:193-199.
DEITCH, E. A., D. XU, L. FRANKO, A. AYALA, and I. H. CHAUDRY. 1994. Evidence favoring the role of the gut as a cytokine-generating organ in rats subjected to hemorrhagic shock. Shock. 1:141-145.
DEITCH, E. A. 1992. Multiple organ failure: Pathophysiology and potential future therapy. Ann. Surg. 216:117-134.
BONE, R. C. 1991 The pathogenesis of sepsis. Ann. Int. Med. 115:457-469.
DEMARIA, E., and J. M. DALTON. 1997. Bacterial translocation and the release of endotoxin and cytokines following trauma. In: Cytokines in Trauma and Hemorrhage, Sugerman et al. editors. Chapman and Hall 43-61.
TERAGUCHI, S., K. SHIN, T. OGATA, M. KINGAKU, A. KAINO, H. MIYAUCHI, Y. FUKUWATARI, and S. SHIMAMURA. 1995. Orally administered bovine lactoferrin inhibits bacterial translocation in mice fed bovine milk. Appl. Env. Microbiol. 61:4131-4144.
TERAGUCHI, S., K. SHIN, K. OZAWA, S. NAKAMURA, Y. FUKUWATARI, S. TSUYUKI, H. NAMIHIRA, and S. SHIMAMURA. 1995. Bacteriostatic effect of orally administered bovine lactoferrin on proliferation of Clostridium species in the gut of mice fed bovine milk. Appl. Env. Microbiol. 61:501-506.
MYERS, I., and D. JOHNSON. 1998. The nonspecific inflammatory response to injury. Can. J. Anaesth. 45:871-879.
STOGAUS, R., and M. G. KING. 1995. Is oral levamisole immunostimulation in rats mediated by reduced levels of free plasma corticosterone? Int. J. Immunopharmac. 17:635-640.
SYMOENS, J., and M. ROSENTHAL. 1977. Levamisol in the modulation of the immune response: the current experimental and clinical state. J. Reticuloendoth. Soc. 21:175-184.
ZIMECKI, M., A. WLASZCZYK, P. CHENEAU, A. S. BRUNEL, J. MAZURIER, G. SPIK, and A. KUBLER. 1998. Immunoregulatory effects of nutritional preparation containing bovine lactoferrin taken orally by healthy individuals. Arch. Immunol. Ther. Exp. 46:231-240.
BLANQUE, R., C. MEAKIN, S. MILLET, and C. R. GARDNER, 1996. Hypothermia as an indicator of the acute effects of lipopolysaccharides: comparison with serum levels of IL-1b, IL-6 and TNFa. Gen. Pharmac. 27:973-977.
KOZAK, W., H. ZHENG, C. A. CONN, D. SOSZYNSKI, L. X. T. VAN DER PLOEG, and M. J. KLUGER. 1995. Thermal and behavioral effects of lipopolysaccharide and influenza in interleukin-1bdeficient mice. Am. J. Physiol. 269:R969-R977.
KOZAK, W., C. A. CONN, J. J. KLIR, G. H. W. WONG, and M. J. KLUGER. 1995. TNF soluble receptor and antiserum against TNF enhance lipopolysaccharide fever in mice Am. J. Physiol. 269:R23-R29.
KOZAK, W., C. A. CONN, and M. J. KLUGER. 1994. Lipopolysaccharide induces fever and depresses locomotor activity in unrestrained mice Am. J. Physiol. 266:R125-135.
BLANQUE, R., C. MEAKIN, S. MILLER and C. R. GARDNER. 1995. Hypothermia as an indicator of the acute effects of lipopolysaccharides: Comparison with serum level of IL 1b, IL 6 and TNFa. Gen. Pharmac. 27:973-977.
KRUZEL, M. K., Y. HARARI, C. YING, A. C. CASTRO. 1998. Role of lactoferrin in development of systemic inflammatory response syndrome (SIRS). 2nd International Conference “Progress in Intensive Care Medicine” Wroclaw, Poland, May, pp. 11-12.
KRUZEL, M., T. ZAGULSKI, and M. ZIMECKI. Lactoferrin and insult-induced metabolic imbalance. Proceedings, 4th International Conference on Lactoferrin: Structure, Function and Applications, K. Shimazaki editor, ICS. (In press.)
ZAGULSKI, T., M. ZIMECKI and M. KRUZEL. Is nitric oxide involved in the protective effect against E. coli generated by lactoferrin in vivo? Proceedings, 4th International Conference on Lactoferrin: Structure, Function and Applications, K. Shimazaki editor ICS. (In press.)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kruzel, M.L., Harari, Y., Chen, CY. et al. Lactoferrin Protects Gut Mucosal Integrity During Endotoxemia Induced by Lipopolysaccharide in Mice. Inflammation 24, 33–44 (2000). https://doi.org/10.1023/A:1006935908960
Issue Date:
DOI: https://doi.org/10.1023/A:1006935908960