Abstract
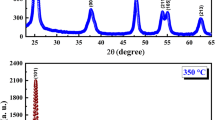
Nanosized TiO2 powder with anatase structure was synthesized by a sol-gel method using TiCl4 ethanol solution as a precursor. The grain size of TiO2 powder was homogenous and was about 10 nm after the precursor was calcined at 500 °C for 1 hour. Anatase TiO2 powder formed after the precursor was calcined at a temperature ranging from 300 °C to 550 °C. The gelatinizing mechanism of TiCl4 in ethanol solution can be described as followings. When mixed with ethanol, TiCl4 reacted with ethanol to form TiCl x (OCH2CH3)4 − x species and HCl gas. During gelatinizing process, TiCl x (OCH2CH3)4 − x species absorbed water from atmosphere to form Ti(OH)4 precursor, which was polymerized to be an inorganic polymer. The formation of inorganic polymer of Ti(OH)4 was intensified with gelatinizing time. In contrast, the organic component was removed from the precursor. The formation of anatase TiO2 can also be promoted by increasing gelatinizing time. The influence of alcohol on the reacting progress and dispersivity was also studied. The size and activity of alcohol molecule were found to have influence on the polymerization and mineralization degree of the precursor and the dispersivity of TiO2 powders.
Similar content being viewed by others
References
D. F. Ollis and H. Al-Ekabi (eds.), “Photocatalytic Purification of Water and Air” (Elsevier, Amsterdam, 1993).
U. Bach, D. Lupo, P. Comte, J. E. Moser, F. Weissortel, J. Salbeck, H. Spreitzer and M. Gratzel, Nature 395 (1998) 583.
B. O'Regan and M. Gratzel, ibid. 353 (1991) 737.
W. A. Zeltner, C. G. Hill and M. A. Anderson, Chemtech 5 (1993) 21.
X. Fu, W. Zeltner and M. A. Anderson, Appl. Catal. B: Environmental (1995) 209.
I. Sopyan, M. Watanabe, S. Murasawa, K. Hashimoto and A. Fujishima, J. Photochemistry and Photobiology A: Chemistry 98 (1996) 79.
E. Pelizzetti and C. Minero, Electrochim. Acta. 38 (1993) 47.
M. Anpo, T. Shima and S. Kodama, J. Phys. Chem. 91 (1987) 4035.
K. Tennakon and I. R. M. Kottegoda, J. Photochemistry and Photobiology A: Chemistry 93 (1996) 79.
M. Anpo, T. Shima and S. Kodama, J. Phys. Chem. 91 (1987) 4305.
C. T. Kresge, M. E. Leonowica and W. J. Roth, Nature 359 (1992) 710.
C. J. Barbe, F. Arendse, P. Comte, M. Jirousek, F. Lenzmann, V. Shklover and M. Gratzel, A. Am. Ceram. Soc. 80(12) (1997) 3157.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhu, Y., Zhang, L., Gao, C. et al. The synthesis of nanosized TiO2 powder using a sol-gel method with TiCl4 as a precursor. Journal of Materials Science 35, 4049–4054 (2000). https://doi.org/10.1023/A:1004882120249
Issue Date:
DOI: https://doi.org/10.1023/A:1004882120249