Abstract
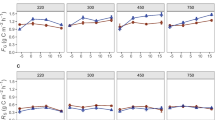
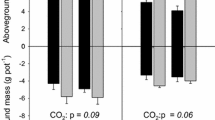
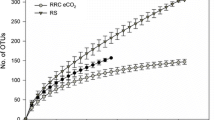
The impact of elevated atmospheric CO2 on qualitative and qua ntitative changes in rhizosphere carbon flow will have important consequences fo r nutrient cycling and storage in soil, through the effect on the activity, biom ass size and composition of soil microbial communities. We hypothesized that mic robial communities from the rhizosphere of Danthonia richardsonii, a n ative C3 Australian grass, growing at ambient and twice ambient CO2 a nd varying rates of low N application (20, 60, 180 kg N ha-1) will be different as a consequence of qualitative and quantitative change in rhizosphere carbon flow. We used the BiologTM system to construct sole carbon source utilisation profiles of these communities from the rhizosphere of D. richardsonii. BiologTM GN and MT plates, the latter to which more ecologically relevant root exudate carbon sources were added, were used to characterise the communities. Microbial communities from the rhizosphere of D. richardsonii grown for four years at twice ambient CO2 had significantly greater utilisation of all carbon sources except those with a low C:N ratio (neutral and acidic amino acids, amides, N-heterocycles, long chain aliphatic acids) than communities from plants grown at ambient CO2. This indicates a change in microbial community composition suggesting that under elevated CO2 compounds with a higher C:N ratio were exuded. Enumeration of microorganisms, using plate counts, indicated that there was a preferential stimulation of fungal growth at elevated CO2 and confirmed that bacterial metabolic activity (C utilisation rates), not population size (counts), were stimulated by additional C flow at elevated CO2. Nitrogen was an additional rate-limiting factor for microbial growth in soil and had a significant impact on the microbial response to elevated CO2. Microbial populations were higher in the rhizosphere of plants receiving the highest N application, but the communities receiving the lowest N application were most active. These results have important implications for carbon turnover and storage in soils where changes in soil microbial community structure and stimulation of the activity of microorganisms which prefer to grow on rhizodeposits may lead to a decrease in the composition of organic matter and result in an accumulation of soil carbon.
Similar content being viewed by others
References
Bazzaz F A 1990 The response of natural ecosystems to the rising global CO2 levels. Annu. Rev. Ecol. Syst. 21, 167–196.
Berntson G M and Woodward F I 1992 The root system architecture and development of Senecio vulgaris in elevated CO2 and drought. Funct. Ecol. 6, 324–333.
BIOLOG 1993 Manual for the Identification of Gram Negative Bacteria. BIOLOG Inc., Hayward, California, U.S.A. 307 p.
Bowler J M and Press M C 1996 Effects of elevated CO2, nitrogen form and concentration on growth and photosynthesis of a fast-and slow-growing grass. New Phytol. 132, 391–401.
Campbell C D, Grayston S J and Hirst D 1997 Use of rhizosphere carbon sources in sole carbon source tests to discriminate soil microbial communities. J. Microbiol. Methods 30, 33–41.
Couteaux M M, Mousseau M, Celerier M L and Bottner P 1991 Increased atmospheric CO2 and litter quality: decomposition of sweet chestnutleaf litter with animal food webs of different complexities. Oikos 61, 54–65
Diaz S, Grime J P, Harris J and McPherson J 1993 Evidence of a feedback mechanism limiting plant response to elevated CO2. Nature 364, 616–617.
Duchein M, Bonicel A and Betsche T 1993 Photosynthetic net CO2 uptake and leaf phosphate concentrations in CO2 enriched clover (Trifolium subterraneum L.) at three levels of phosphate nutrition. J. Exp. Bot. 44, 17–22.
Garland J L 1996a Analytical approaches to the characterisation of samples of microbial communities using patterns of potential C source utilisation. Soil Biol. Biochem. 28, 213–221.
Garland J L 1996b Patterns of potential C source utilisation by rhizosphere communities. Soil Biol. Biochem. 28, 223–230.
Garland J L and Mills A L 1991 Classification and characterisation of heterotrophic microbial communities on the basis of patterns of community-level-sole-carbon-source utilization. Appl. Environ. Microbiol. 57, 2351–2359.
Grayston S J and Campbell C D 1996 Functional biodiversity of microbial communities in the rhizosphere of hybrid larch (Larix eurolepis) and Sitka spruce (Picea sitchensis). Tree Physiol. 16, 1031–1038.
Grayston S J, Wang S, Campbell C D and Edwards A C 1998 Selective influence of plant species on microbial diversity in the rhizosphere. Soil Biol. Biochem. 30, 369–378.
Helal H M and Sauerbeck D 1986 Effect of plant roots on carbon metabolism of soil microbial biomass. Z. Pflanzenernaehr. Bodenkd. 149, 181–188.
Hodge A, Grayston S J and Ord B G 1996 A novel method for characterisation and quantification of plant root exudates. Plant Soil 184, 97–104.
Hodge A, Paterson E, Grayston S J, Campbell C D, Ord B G and Killham K 1998 Characterisation and microbial utilisation of exudate material from the rhizosphere of Lolium perenne grown under CO2 enrichment. Soil Biol. Biochem. 30, 1033–1043.
Holland E A and Coleman D C 1987 Litter placement effects on microbial and organic matter dynamics in an agroecosystem. Ecology 68, 425–433.
Idso K E and Idso S B 1994 Plant responses to atmospheric CO2 enrichment in the face of environmental constraints: a review of the past 10 years research. Agric. For. Meteorol. 69, 153–203.
Jongen M, Fay P and Jones M B 1996 Effects of elevated carbon dioxide and arbuscular mycorrhizal infection on Trifolium repens. New Phytol. 132, 413–423.
Kimball B A 1983 Carbon dioxide and agricultural yield, an assemblage and analysis of 430 prior observations. Agron. J. 75, 779–788.
Kowalenko C G, Ivarson K C and Cameron D R 1978 Effect of moisture content, temperature and nitrogen fertilisation on carbon dioxide evolution from field soils. Soil Biol. Biochem. 10, 417–423.
Körner C and Arnone J A III 1992 Responses to elevated carbon dioxide in artificial tropical ecosystems. Science 257, 1672–1675.
Lekkerkerk L J A, van de Geijn S C and van Veen J A 1990 Effects of elevated atmospheric CO2 levels on the carbon economy of a soil planted with wheat. In Soils and the Greenhouse Effect. Ed. A F Bouwman. pp 423–429. John Wiley & Sons, New York.
Leuken H, Hutchinson W L and Paul E A 1962. The influence of nitrogen on the decomposition of crop residues in the soil. Can. J. Soil Sci. 42, 276–288.
Liljeroth E, van Veen J A and Miller H J 1990 Assimilate translocation to the rhizosphere of two wheat cultivars and subsequent utilization by microorganisms at two soil nitrogen levels. Soil Biol. Biochem. 22, 1015–1021.
Lutze J L and Gifford R M 1995 Carbon storage and productivity of a carbon dioxide enriched nitrogen limited grass sward after one year's growth. J. Biogeog. 22, 227–233.
Lutze J L and Gifford R M 1998 Carbon accumulation, distribution and water use of D. richardsonii swards in response to CO2 and nitrogen supply over four years of growth. J. Biogeog. (Submitted).
Marschner H 1995 Mineral Nutrition of Higher Plants. pp 537–595. Academic Press, London.
Melillo J M 1983 Will increases in atmospheric CO2 concentrations affect decay processes? Ecosys. Center Ann. Rep., Marine Biol. Lab., Woods Hole, Massachusetts. pp 10–11.
Melillo J M, Callaghan T V, Woodward F I, Salati E and Sinha SK 1990 Effects on ecosystems. In Climate Change: The IPCC Scientific Assessment. Eds. J T Houghton, G J Jenkins and J J Ephraums. pp 283–310.
Merckx R, Dijkstra A, den Hartog A and van Veen J A 1987 Production of root-derived material and associated microbial growth in soil at different nutrient levels. Biol. Fertil. Soils 5, 126–132.
Newbery R M and Wolfenden J 1996 Effects of elevated CO2 and nutrient supply on the seasonal growth and morphology of Agrostis capillaris. New Phytol. 132, 403–411.
Norby R J, O'Neill E G, Hood W G and Luxmoore R J 1987 Carbon allocation, root exudation and mycorrhizal colonization of Pinus echinata seedlings grown under CO2 enrichment. Tree Physiol. 3, 203–210.
Norby R J, O'Neill E G and Wullschleger S D 1994 Belowground responses to atmospheric carbon dioxide in forests. In Carbon Forms and Functions in Forest Soils. Eds. W W McFee and J M Kelly. pp 397–418. Soil Sci. Soc. Am., Madison, WI.
O'Neill E G 1994 Responses of soil biota to elevated atmospheric carbon dioxide. Plant Soil 165, 55–65.
Owensby C E, Coyne P I and Auen L 1993 Nitrogen and phosphorus dynamics of a tallgrass prairie ecosystem exposed to elevated carbon dioxide. Plant Cell Environ. 16, 843–850.
Patterson D T 1986 Responses of soybean and three C4 grass weeds to CO2 enrichment during drought. Weed Science 34, 203–210.
Rice C W, Garcia F O, Hampton C O and Owensby C E 1994 Soil microbial response in tallgrass prairie to elevated CO2. Plant Soil 165, 65–74.
Rogers H H, Runion G B and Krupa S V 1994 Plant responses to atmospheric CO2 enrichment with emphasis on roots and the rhizosphere. Environ. Pollut. 83, 155–189.
Runion G B, Curl E A, Rogers A A, Backman P A, Rodriguez-Kabana R and Helms B E 1994 Effects of CO2 enrichment on microbial populations in the rhizosphere and phyllosphere of cotton. Agric. For. Met. 70, 117–130.
Siegenthaler U 1990 El Niño and atmospheric CO2. Nature 345, 295–296.
Söderström B, Bååth E and Lundgren B 1983 Decrease in soil microbial activity and biomass owing to nitrogen amendments. Can. J. Microbiol. 29, 1500–1506.
Vahjen W, Munch J C and Tebbe C C 1995 Carbon source utilisation of soil extracted microorganisms as a tool to detect the effects of soil supplemented with genetically-engineered and non-engineered Corynebacterium glutamicum and a recombinant peptide at the community level. FEMS Microbiol. Ecol. 18, 317–328.
van Veen J A, Liljeroth E, Lekkerkerk L J A and van de Geijn S C 1991 Carbon fluxes in plant-soil systems at elevated atmospheric CO2 levels. Ecological Appl. 1, 175–181.
Ward S M and Strain B R 1986 Response of two old field perennials to interactions of CO2 enrichment and drought stress. Am. J. Bot. 73, 1486–1491.
Wardle D A 1992 A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biol. Rev. 67, 321–358.
Whipps J M 1985 Effect of CO2 concentration on growth, carbon distribution and loss of carbon from the roots of maize. J. Exp. Bot. 36, 644–651.
Winding A K 1994 Fingerprinting bacterial soil communities with BIOLOG microtiter plates. In Beyond the Biomass Compositional and Functional Analysis of Soil Microbial Communities. Eds. K Ritz, J Dighton and K E Giller. pp 85–94. Wiley, Chichester.
Zak D R, Pregitzer K S, Curtis P, Teeri J A, Fogel R and Randlett D L 1993 Elevated atmospheric CO2 and feedback between carbon and nitrogen cycles in forested ecosystems. Plant Soil 151, 105–117.
Zak D R, Ringelberg D B, Pregitzer K S, Randlett D L, White D C and Curtis P S 1996 Soil microbial communities beneath Populus grandidentata grown under elevated atmospheric CO2. Ecol. Appl. 6, 257–262.
Zak J C, Willig M R, Moorhead D L, Wildman H G 1994 Functional diversity of microbial communities: A quantitative approach. Soil Biol. Biochem. 26, 1101–1108.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Grayston, S.J., Campbell, C.D., Lutze, J.L. et al. Impact of elevated CO2 on the metabolic diversity of microbial communities in N-limited grass swards. Plant and Soil 203, 289–300 (1998). https://doi.org/10.1023/A:1004315012337
Issue Date:
DOI: https://doi.org/10.1023/A:1004315012337