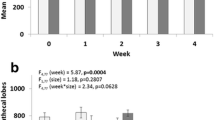
Abstract
The Asian tiger mosquito, Aedes albopictus (Skuse) and the yellow fever mosquito, Aedes aegypti (Linnaeus) are medically important species that vector several arboviruses. Globally, populations of both species experience (and are sensitive to) photoperiodic variations. The present study aims to test if photoperiod regimes affect the population performance of Ae. albopictus and Ae. aegypti. Since mosquitoes have sex-specific strategies to maximize fitness, we also tested the hypothesis that Ae. albopictus and Ae. aegypti would exhibit differences in the male and female response (sexually dimorphic response) to various photoperiod treatments. We reared cohorts of first instar larvae to adulthood in three photoperiod treatments: short day (10 h light), control (12 h light) and long day (14 h light). We measured and compared survival to adulthood, population growth, development time of males and females, and wing length across treatments. Although we detected no effects of photoperiod on the population performance of both species, we found evidence of a sexual dimorphic response to photoperiod in Ae. albopictus, but not in Ae. aegypti, with Ae. albopictus females being more sensitive to variations in photoperiod. The observed differences between sexes of Ae. albopictus are consistent with sex-specific developmental constraints. The absence of a sexually dimorphic response to photoperiod in Ae. aegypti can be attributed to different strategies evolved in this species to prepare for unfavourable conditions associated with shorter day length. We discuss the ecological and medical implications of our findings.
Similar content being viewed by others
References
Alto B. W. and Juliano S. A. (2001) Precipitation and temperature effects on populations of Aedes albopictus (Diptera: Culicidae): Implications for range expansion. Journal of Medical Entomology 38, 646–656.
Alto B. W., Lampman R. L., Kesavaraju B. and Muturi E. J. (2013) Pesticide-induced release from competition among competing Aedes aegypti and Aedes albopictus (Diptera: Culicidae). Journal of Medical Entomology 50, 1240–1249.
Alto B. W. and Lounibos L. P. (2013) Vector competence for arboviruses in relation to the larval environment of mosquitoes, pp. 81–101. In Ecology of Parasite- Vector Interactions Vol. 3. (edited by W. Takken and C. J. M. Koenraadt). Wageningen Academic Publishers, The Netherlands.
Alto B. W., Lounibos L. P., Mores C. N. and Reiskind M. H. (2008a) Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proceedings of the Royal Society B: Biological Sciences 275, 463–471.
Alto B. W., Reiskind M. H. and Lounibos L. P. (2008b) Size alters susceptibility of vectors to dengue virus infection and dissemination. American Journal of Tropical Medicine and Hygiene 79, 688–695.
Anderson J. F. (1968) Influence of photoperiod and temperature on the induction of diapause in Aedes atropalpus (Diptera: Culicidae). Entomologia Experi-mentalis et Applicata 11, 321–330. doi:10.1111/j.1570-7458.1968.tb02061.x.
Bedhomme S., Agnew P., Sidobre C. and Michalakis Y. (2003) Sex-specific reaction norms to intraspecific larval competition in the mosquito Aedes aegypti. Journal of Evolutionary Biology 16, 721–730. doi:10.1046/j.1420-9101.2003.00576.x.
Bevins S. N. (2008) Invasive mosquitoes, larval competition, and indirect effects on the vector competence of native mosquito species (Diptera: Culicidae). Biological Invasions 10, 1109–1117. doi:10.1007/s10530-007-9188-8.
Brady O. J., Johansson M. A. Guerra C. A., Bhatt S. Golding N., Pigott D. M., Delatte H. Grech M. G., Leisnham P. T., Maciel-de-Freitas R., Styer L. M., Smith D. L., Scott T. W., Gething P. W. and Hay S. I. (2013) Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings. Parasites & Vectors 6, 351. doi:10.1186/1756-3305-6-351.
Briegel H. (1990) Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. Journal of Insect Physiology 36, 165–172.
Carrington L. B., Armijos M. V., Lambrechts L., Barker C. M. and Scott T. W. (2013a) Effects of fuctuating daily temperatures at critical thermal extremes on Aedes aegypti life-history traits. PLoS One 8(3), e58824. doi:10.1371/journal.pone.0058824.
Carrington L. B., Seifert S.N., Armijos M. V., Lambrechts L. and Scott T. W. (2013b) Reduction of Aedes aegypti vector competence for dengue virus under large temperature fuctuations. American Journal of Tropical Medicine and Hygiene 88, 689–697. doi:10.4269/ajtmh.12-0488.
Costanzo K. S., Kesavaraju B. and Juliano S. A. (2005) Condition-specific competition in container mosquitoes: the role of noncompeting life-history stages. Ecology 86, 3289–3295.
Costanzo K. S., Muturi E. J. and Alto B. W. (2011a) Trait- mediated effects of predation across life-history stages in container mosquitoes. Ecological Entomology 36, 605–615. doi:10.1111 /j.1365-2311.2011.01302.x.
Costanzo K. S., Muturi E. J., Lampman R. L. and Alto B. W. (2011b) The effects of resource type and ratio on competition with Aedes albopictus and Culex pipiens (Diptera: Culicidae). Journal of Medical Entomology 48, 29–38.
Costanzo K., Schelble Jerz S. and Keenan M. (2015) The effect of photoperiod on life history and blood-feeding activity in Aedes albopictus and Aedes aegypti (Diptera: Culicidae). Journal of Vector Ecology 40, 164–171. doi:10.1111 /jvec.12146.
Darsie R. F. Jr. and Ward R. A. (2005) Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. University of Florida Press, Gainesville, FL, USA. 400 pp.
Delatte H., Gimonneau G., Triboire A. and Fontenille D. (2009) Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. Journal of Medical Entomology 46, 33–41.
Dye C. (1986) Vectorial capacity: Must we measure all its components? Parasitology Today 2, 203–209.
Eisen L. and Moore C. G. (2013) Aedes (Stegomyia) aegypti in the continental United States: A vector at the cool margin of its geographic range. Journal of Medical Entomology 50, 467–478.
Enserink M. (2008) Entomology. A mosquito goes global. Science 320, 864–866. doi:10.1126/science.320.5878.864.
Farjana T. and Tuno N. (2013) Multiple blood feeding and host-seeking behavior in Aedes aegypti and Aedes albopictus (Diptera: Culicidae). Journal of Medical Entomology 50, 838–846.
Grard G., Caron M., Mombo I. M., Nkoghe D., Mboui Ondo S., Jiolle D., Fontenille D., Paupy C. and Leroy E. M. (2014) Zika virus in Gabon (Central Africa)-2007: A new threat from Aedes albop-ictus? PLoS Neglected Tropical Diseases 8(2), e2681. doi:10.1371/journal.pntd.0002681.
Gratz N. G. (2004) Critical review of the vector status of Aedes albopictus. Medical and Veterinary Entomology 18, 215–227. doi:10.1111/j.0269-283X.2004.00513.x.
Grill C. P. and Juliano S. A. (1996) Predicting species interaction based on behavior: Predation and competition in container-dwelling mosquitoes. Journal of Animal Ecology 65, 63–76. doi:10.2307/5700.
Griswold M. W. and Lounibos L. P. (2005) Competitive outcomes of aquatic container Diptera depend on predation and resource levels. Annals of the Entomological Society of America 98, 673–681.
Hahn D. A. and Denlinger D. L. (2007) Meeting the energetic demands of insect diapause: Nutrition storage and utilization. Journal of Insect Physiology 53, 760–773.
Hawley W.A. (1988) The biology of Aedes albopictus. Journal of the American Mosquito Control Association (Suppl.) 4, 1–40.
Hawley W. A., Reiter P., Copeland R. S., Pumpuni C. B. and Craig G. B. Jr. (1987) Aedes albopictus in North America: Probable introduction in used tires from northern Asia. Science 236, 1114–1116. doi:10.1126/science.3576225.
Hayes E.B. (2009) Zika virus outside Africa. Emerging Infectious Disease 15, 1347–1350.
Jordan R. G. and Bradshaw W. E. (1978) Geographic variation in the photoperiodic response of the Western tree-hole mosquito, Aedes sierrensis. Annals of the Entomological Society of America 71, 487–490. doi:10.1093/aesa/71.4.487.
Joy T. K., Arik A. J., Corby-Harris V., Johnson A. A. and Reihle M. A. (2010) The impact of larval and adult dietary restriction on lifespan, reproduction and growth in the mosquito Aedes aegypti. Experimental Gerontology 45, 685–690. doi:10.1016/j.exger.2010.04.009.
Juliano S. A. (1998) Species introduction and replacement among mosquitoes: Interspecific resource competition or apparent competition? Ecology 79, 255–268.
Juliano S. A. and Lounibos L. P. (2005) Ecology of invasive mosquitoes: Effects on resident species and on human health. Ecology Letters 8, 558–574.
Juliano S. A., O’Meara G. F., Morrill J. R. and Cutwa M. M. (2002) Desiccation and thermal tolerance of eggs and coexistence of competing mosquitoes. Oecologia 130, 458–469.
Kleckner C. A., Hawley W. A., Bradshaw W. E., Holzapfel C. M. and Fisher I. J. (1995) Protandry in Aedes sier-rensis: the significance of temporal variation in female fecundity. Ecology 76, 1242–1250. doi:10.2307/1940931.
Lanciani C. A. and Anderson J. F. (1993) Effect of pho-toperiod on longevity and metabolic rate in Anopeheles quadrimaculatus. Journal of the American Mosquito Control Association 9, 158–163.
Leisnham P. T., Towler L. and Juliano S. A. (2011) Geographic variation of photoperiodic diapause but not adult survival or reproduction of the invasive mosquito Aedes albopictus (Diptera: Culicidae) in North America. Annals of the Entomological Socierty of America 104, 1309–1318.
Livdahl T. P. and Sugihara G. (1984) Non-linear interactions of populations and the importance of estimating per capita rates of change. Journal of Animal Ecology 53, 573–580.
Livdahl T. P. and Willey M. S. (1991) Prospects for an invasion: Competition between Aedes albopictus and native Aedes triseriatus. Science 253, 189–191. doi:10.1126/science.1853204.
Lounibos L. P. (2002) Invasions by insect vectors of human disease. Annual Review of Entomology 47, 233–266. doi:10.1146/annurev.ento.47.091201.145206.
Lounibos L. P., Escher R. L., and Lourenço-de-Oliveira R. (2003) Asymmetric evolution of photoperiodic dia pause in temperate and tropical invasive populations of Aedes albopictus (Diptera: Culicidae). Annals of the Entomological Society of America 96, 512–518.
Lounibos L. P., Suárez S., Menéndez Z., Nishimura N., Escher R. L., O’Connell S. M. and Rey J. R. (2002) Does temperature affect the outcome of larval competition between Aedes aegypti and Aedes albopictus? Journal of Vector Ecology 27, 86–95.
Mitchell C. J. (1995) Geographic spread of Aedes albopictus and potential for involvement in arbovirus cycles in the Mediterranean Basin. Journal of Vector Ecology 20, 44–58.
Mogi M., Miyagi I., Abadi K. and Syafruddin (1996) Inter-and intraspecific variation in resistance to desiccation by adult Aedes (Stegomyia) spp. (Diptera: Culicidae) from Indonesia. Journal of Medical Entomology 33, 53–57.
Mohammed A., and Chadee D. D. (2011) Effects of different temperature regimens on the development of Aedes aegypti (L.) (Diptera: Culicidae) mosquitoes. Acta Tropica 119, 38–43.
Mori A., Oda T. and Wada Y. (1981) Studies on the egg diapause and overwintering of Aedes albopictus in Nagasaki. Tropical Medicine 23, 79–90.
Murrell E. G. and Juliano S. A. (2008) Detritus type alters the outcome of interspecific competition between Aedes aegypti and Aedes albopictus (Diptera: Culicidae). Journal of Medical Entomology 45, 375–383.
Muturi E. J., Blackshear M. Jr. and Montgomery A. (2012) Temperature and density-dependent effects of larval environment on Aedes aegypti competence for an alphavirus. Journal of Vector Ecology 37, 154–161. doi:10.1111/j.1948-7134.2012.00212.x.
Nylin S. and Gotthard K. (1998) Plasticity in life-history traits. Annual Review of Entomology 43, 63–83.
Padmanabha H., Bolker B., Lord C. C., Rubio C. and Lounibos L. P. (2011) Food availability alters the effects of larval temperature on Aedes aegypti growth. Journal of Medical Entomology 48, 974–984.
Paupy C., Ollomo B., Kamgang B. Moutailler S., Rousset D., Demanou M., Hervé J.-P., Leroy E. and Simard F. (2010) Comparative role of Aedes albopictus and Aedes aegypti in the emergence of dengue and chikungunya in Central Africa. Vector-Borne and Zoonotic Diseases 10, 259–266.
Pumpuni C. B., Knepler J. and Craig G. B. Jr. (1992) Influence of temperature and larval nutrition on diapause inducing photoperiod in Aedes albopictus. Journal of the American Mosquito Control Association 8, 223–227.
Reiskind M. H. and Lounibos L. P. (2013) Spatial and temporal patterns in abundance of Aedes aegypti L. (Stego-myia aegypti) and Aedes albopictus (Skuse) [Stegomyia al-bopictus (Skuse)] in southern Florida. Medical and Veterinary Entomology 27, 421–429. doi:10.1111 /mve.12000.
Reiter P. (2010) Yellow fever and dengue: A threat to Europe? Eurosurveillance 15(10), pii=19509. Available online: https://doi.org/www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19509.
Rezza G. (2012) Aedes albopictus and the reemergence of dengue. BMC Public Health 12, 72. https://doi.org/doi.org/10.1186/1471-2458-12-72.
Richards S. L., Anderson S. L. and Alto B. W. (2012) Vector competence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) for dengue virus in the Florida Keys. Journal of Medical Entomology 49, 942–946.
Roiz D., Rosà R., Arnoldi D. and Rizzoli A. (2010) Effects of temperature and rainfall on the activity and dynamics of host-seeking Aedes albopictus females in northern Italy. Vector-Borne and Zoonotic Diseases 10, 811–816.
Scheiner S. M. (2001) MANOVA. Multiple response variables and multispecies interactions, pp. 99–133. In Design and Analysis of Ecological Experiments, 2nd ed. (edited by S. M. Scheiner and J. Gurevitch). Oxford University Press, Oxford, United Kingdom.
Steinwascher K. (1982) Relationship between pupal mass and adult survivorship and fecundity for Aedes aegypti. Environmental Entomology 11, 150–153.
Tabachnick W. J. (1991) Evolutionary genetics and arthropod-borne disease: The yellow fever mosquito. American Entomologist 37, 14–24.
Tauber M. J., Tauber C. A. and Masaki S. (1985) Seasonal Adaptations of Insects. Oxford University Press, New York, New York. 426 pp.
Teder T. and Tammaru T. (2005) Sexual size dimorphism within species increases with body size in insects. Oikos 108, 321–334.
Thomas S. M., Obermayr U., Fischer D., Kreyling J. and Beierkuhnlein C. (2012) Low-temperature threshold for egg survival of a post-diapause and non-diapause European aedine strain, Aedes albopictus (Diptera: Culicidae). Parasites & Vectors 5, 100. doi:10.1186/1756-3305-5-100.
Tsunoda T., Cuong T. C., Dong T. D., Yen N. T., Le N. H., Phong T. V. and Minakawa N. (2014) Winter refuge for Aedes aegypti and Ae. albopictus mosquitoes in Hanoi during winter. PLoS One 9(4) e95606. doi:10.1371/journal.pone.0095606.
Urbanski J. M., Benoit J. B., Michaud M. R., Denlinger D. L. and Armbruster P. (2010) The molecular physiology of increased egg desiccation resistance during diapause in the invasive mosquito, Aedes albopictus. Proceedings of the Royal Society B: Biological Sciences 277, 2683–2692. doi:10.1098/rspb.2010.0362.
Vega-Rúa A., Zouache K., Girod R., Failloux A. B. and Lourenço-de-Oliveira R. (2014) High level of vector competence of Aedes aegypti and Aedes albopictus from ten American countries as a crucial factor in the spread of chikungunya virus. Journal of Virology 88, 6294–6306. doi:10.1128/JVI.00370-14.
Waldock J., Chandra N. L., Lelieveld J., Proestos Y., Michael E., Christophides G. and Parham P. E. (2013) The role of environmental variables on Aedes albopictus biology and chikungunya epidemiology. Pathogens and Global Health 107, 224–241. doi:10.1179/2047773213Y.0000000100.
West-Eberhard M. J. (2003) Developmental Plasticity and Evolution. Oxford University Press, New York. 816 pp.
Westbrook C. J., Reiskind M. H., Pesko K. N., Greene K. E. and Lounibos L. P. (2010) Larval environmental temperature and the susceptibility of Aedes albopictus Skuse (Diptera: Culicidae) to chikungunya virus. Vector-Borne and Zoonotic Diseases 10, 241–247. doi:10.1089/vbz.2009.0035.
Wormington J. D. and Juliano S. A. (2014) Sexually dimorphic body size and development time plasticity in Aedes mosquitoes (Diptera: Culicidae). Evolutionary Ecology Research 16, 223–234.
Yee D. A., Juliano S. A. and Vamosi S. M. (2012) Seasonal photoperiods alter developmental time and mass of an invasive mosquito, Aedes albopictus (Diptera: Culicidae), across its north-south range in the United States. Journal of Medical Entomology 49, 825–832.
Yee D. A., Kaufman M. G. and Juliano S. A. (2007) The significance of ratios of detritus types and micro-organism productivity to competitive interactions between aquatic insect detritivores. Journal of Animal Ecology 76, 1105–1115. doi:10.1111/j.1365-2656.2007.01297.x.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Costanzo, K.S., Dahan, R.A. & Radwan, D. Effects of photoperiod on population performance and sexually dimorphic responses in two major arbovirus mosquito vectors, Aedes albopictus and Aedes aegypti (Diptera: Culicidae). Int J Trop Insect Sci 36, 177–187 (2016). https://doi.org/10.1017/S1742758416000163
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1017/S1742758416000163